Alcon Vision Suite
A seamless ecosystem designed to power your practice by bringing together the industry’s most comprehensive OR suite with clinical diagnostics and cloud-based solutions, plus first-class training and services.1

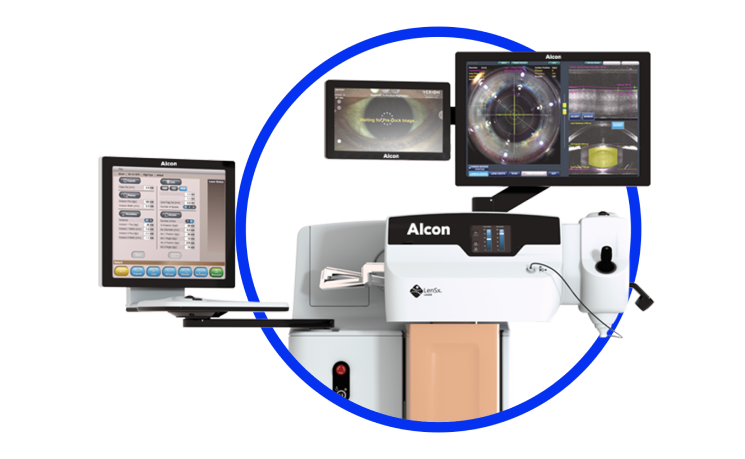
ARGOS® Biometer with Image Guidance
The smarter planning solution that keeps efficiency and accuracy flowing through your clinic

LuxOR® Revalia™
The fastest growing ophthalmic microscope in the United States7*
*Based on year-over-year new equipment install data. Data current as of March 2021.

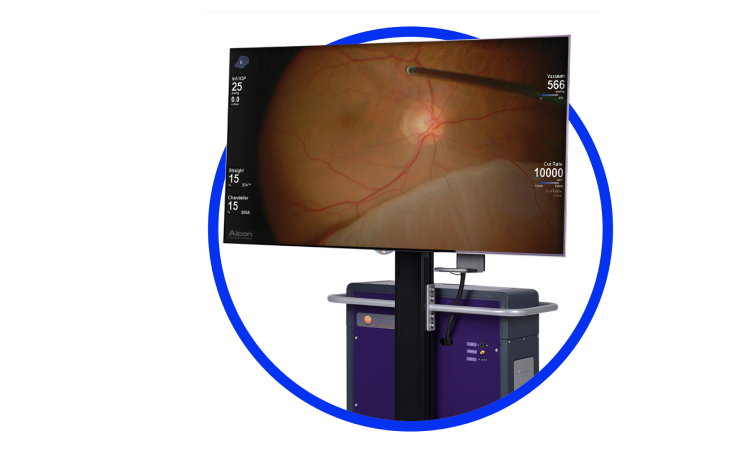
NGENUITY® 3D Visualization System
Redefine what’s possible with true digital visualization8
CENTURION® Vision System with ACTIVE SENTRY™
Helps to offer a new baseline of safety, consistency and control to every cataract patient2-5

ORA SYSTEM® powered by AnalyzOR™ Technology
Get real-time data verification and guidance9

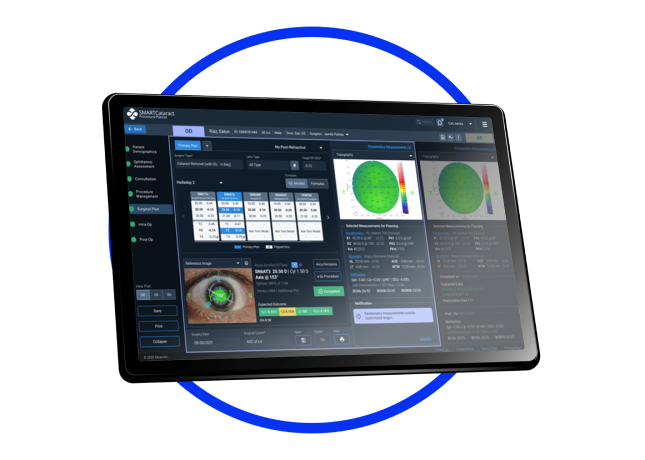
SMART Solutions
Digital Health Solutions to help your practice work smarter, connecting the clinic to the OR seamlessly 10-12
Alcon Experience Academy
For relevant training content from industry thought leaders
References
- Market Scope. 2020 Ophthalmic Diagnostic Equipment Report: A Global Analysis for 2019 to 2025.
- CENTURION® Vision System Operator's Manual.
- Alcon Data on File, 2017.
- Thorne A, Dyk DW, Fanney D, Miller KM. Phacoemulsifier occlusion break surge volume reduction. J Cataract Refract Surg. 2018 Dec;44(12):1491-1496.
- Aravena C, Dyk DW, Thorne A, Fanney D, Miller KM. Aqueous volume loss associated with occlusion break surge in phacoemulsifiers from 4 different manufacturers. J Cataract Refract Surg. 2018 Jul;44(7):884-888.
- Alcon Data on File, 2018.
- Alcon Data on File, 2021.
- NGENUITY® 3D Visualization System Operator’s Manual.
- ORA SYSTEM® with VerifEye+™ Operator’s Manual.
- Alcon Data on File, 2020.
- Van Vliet, EJ, et al. Exploring the relation between process design and efficiency in high-volume cataract pathways from a lean thinking perspective. International Journal for Quality in Health Care. 2011; 23(1):83-93
- Hakansson I, et al. Data reliability and structure in the Swedish National Cataract Register. Acta Ophthalmologica Scandinavia. 2001; 79(5):518-523.


