Clareon® PanOptix® Trifocal IOL
20/20 Near, Intermediate, and Distance Vision is now possible1
Clareon® PanOptix® Trifocal IOL
20/20 Near, Intermediate, and Distance Vision is now possible1

The first and only trifocal lens in the United States
The latest advancements in lens technology enable the Clareon® PanOptix® IOL to deliver a full range of vision and exceptional clarity.1,2*
The first and only trifocal lens in the United States
The latest advancements in lens technology enable the Clareon® PanOptix® IOL to deliver a full range of vision and exceptional clarity.1,2*
ENLIGHTEN® Optical Technology
The Clareon® PanOptix® IOL is an advanced trifocal IOL equipped with ENLIGHTEN® Optical Technology—a proprietary design that optimizes intermediate vision without compromising exceptional near and distance vision.3,4
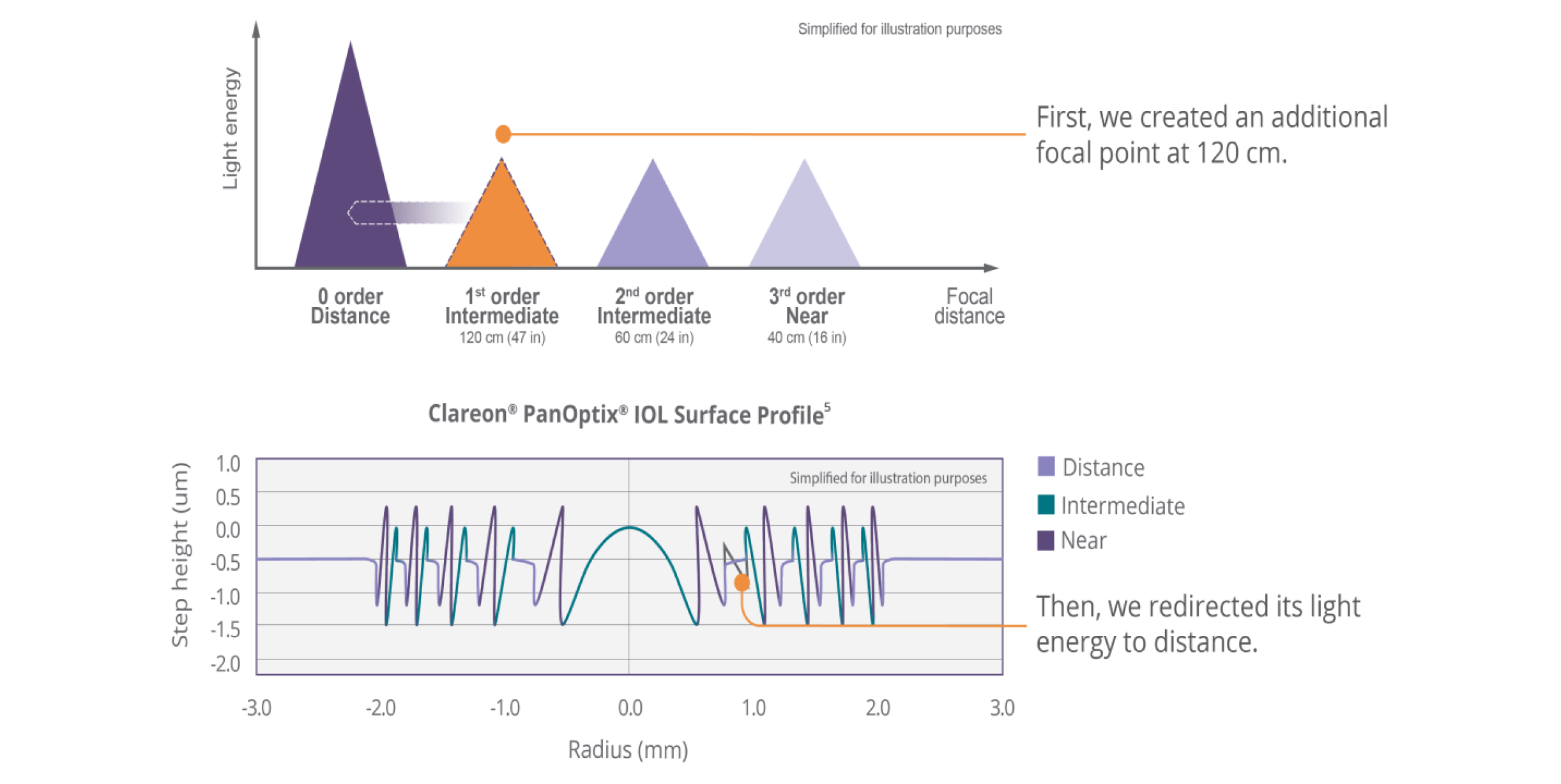
See the difference Clareon®PanOptix® provides your patients
Optimized light energy distribution
| 88% total light utilization at a 3 mm pupil size6 (Light allocation: 50% distance, 25% intermediate, 25% near) |
| Reduces dependence on pupil size6,7 with a 4.5 mm diffractive zone |
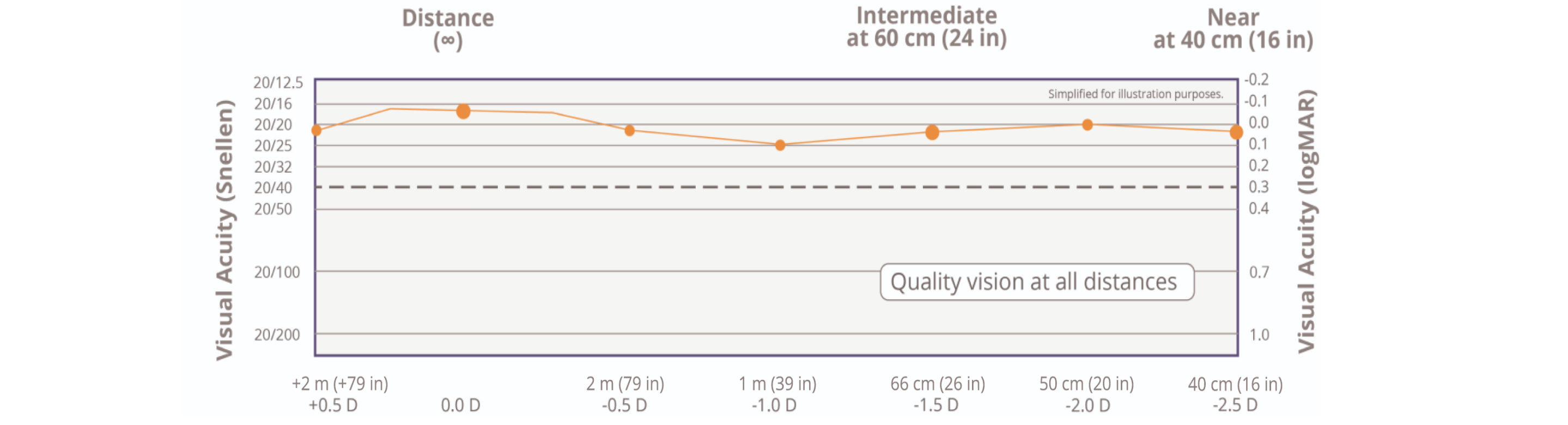
20/20 near, intermediate, and distance vision is now possible1†‡
Hear what surgeons are saying about the Clareon® PanOptix® IOL
Explore additional resources for Clareon® PanOptix® IOLs
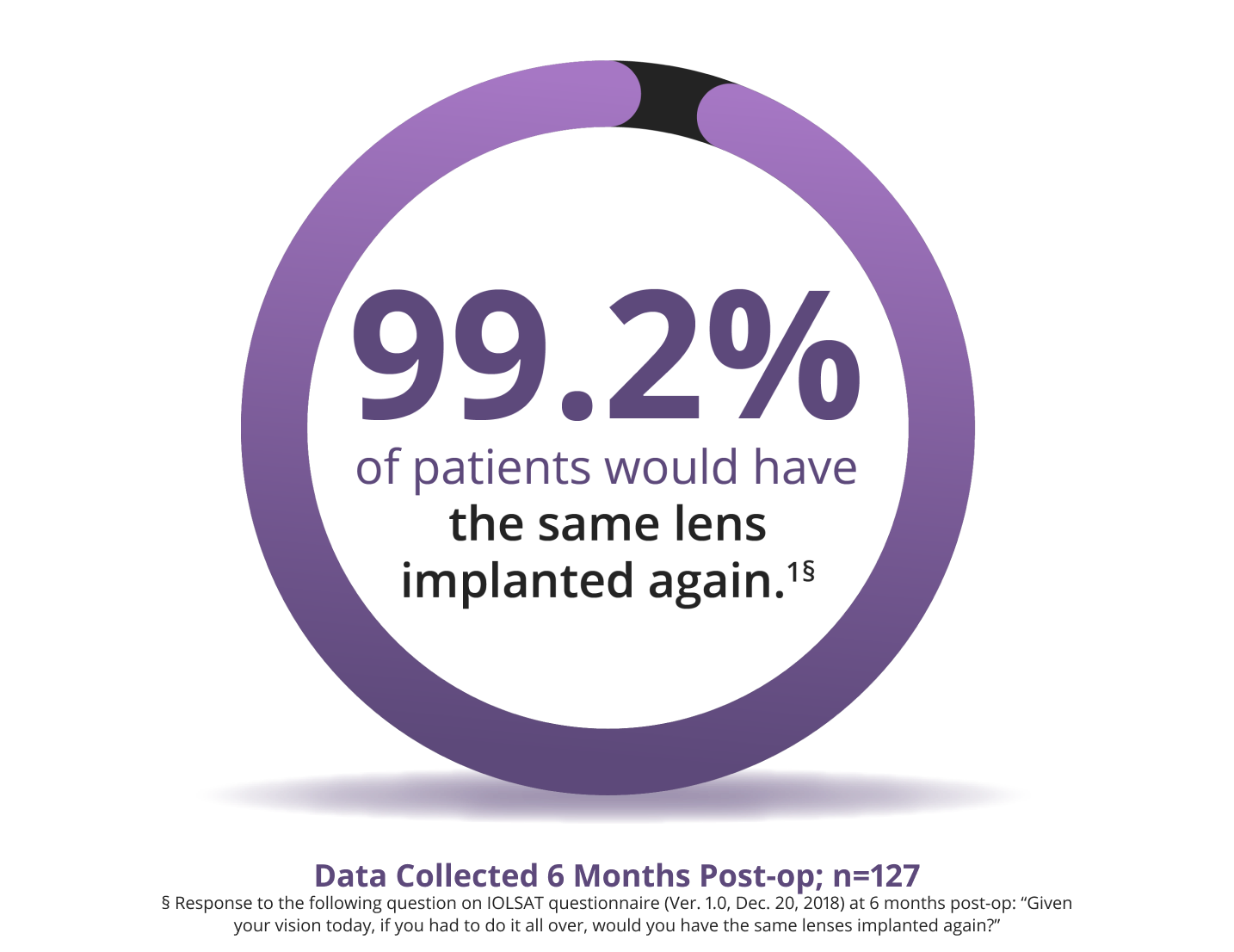
Proven Patient Satisfaction
Patients love their results with the Clareon® PanOptix® IOL1
Hear about the impact the Clareon® PanOptix® IOL is having on patients
Share our product brochure with your patients to communicate the Clareon® difference


AVAILABLE ON CLAREON®: ALCON'S MOST ADVANCED IOL BIOMATERIAL TO DATE



* Based on in vitro examinations of glistenings, surface haze and SSNGs.
† Based on mean value of binocular defocus curve at near, intermediate, and distance at 6 months (n=127).
‡ Snellen VA was converted from logMAR VA. A Snellen notation of 20/20-2 or better indicates a logMAR VA of 0.04 or better, which means 3 or more of the5 Early Treatment Diabetic Retinopathy Study chart letters in the line were identified correctly.
IMPORTANT PRODUCT INFORMATION - CLAREON® PANOPTIX® FAMILY OF TRIFOCAL HYDROPHOBIC IOLS
CAUTION: Restricted by law to sale by or on the order of a physician.
INDICATIONS: The Clareon® PanOptix® Family of Trifocal Hydrophobic IOLs include Clareon® PanOptix® and Clareon® Panoptix® Toric and are indicated for primary implantation in the capsular bag in the posterior chamber of the eye for the visual correction of aphakia in adult patients, with less than 1 diopter of pre-existing corneal astigmatism, in whom a cataractous lens has been removed. The lens mitigates the effects of presbyopia by providing improved intermediate and near visual acuity, while maintaining comparable distance visual acuity with a reduced need for eyeglasses, compared to a monofocal IOL. In addition, the Clareon® PanOptix® Toric Trifocal IOL is indicated for the reduction of residual refractive astigmatism.
WARNINGS / PRECAUTIONS: Careful preoperative evaluation and sound clinical judgment should be used by the surgeon to decide the risk/benefit ratio before implanting a lens in a patient with any of the conditions described in the Directions for Use labeling. Physicians should target emmetropia, and ensure that IOL centration is achieved. For the Clareon® PanOptix® Toric Trifocal IOLs, the lens should not be implanted if the posterior capsule is ruptured, if the zonules are damaged, or if a primary posterior capsulotomy is planned. Rotation can reduce astigmatic correction; if necessary lens repositioning should occur as early as possible prior to lens encapsulation. Some visual effects may be expected due to the superposition of focused and unfocused multiple images. These may include some perceptions of halos, radial lines around point sources of light (starbursts) under nighttime conditions, or glare, as well as other visual symptoms. As with other multifocal IOLs, there is a possibility that visual symptoms may be significant enough that the patient will request explant of the multifocal IOL. A reduction in contrast sensitivity as compared to that expected with a monofocal IOL may be experienced by some patients and may be more prevalent in low lighting conditions. Therefore, patients implanted with multifocal IOLs should exercise caution when driving at night or in poor visibility conditions. Patients should be advised that unexpected outcomes could lead to continued spectacle dependence or the need for secondary surgical intervention (e.g., intraocular lens replacement or repositioning). As with other multifocal IOLs, patients may need glasses when reading small print or looking at small objects. Posterior capsule opacification (PCO), may significantly affect the vision of patients with multifocal IOLs sooner in its progression than patients with monofocal IOLs. Prior to surgery, physicians should provide prospective patients with a copy of the Patient Information Brochure available from Alcon informing them of possible risks and benefits associated with the IOLs.
ATTENTION: Reference the Directions for Use labeling for each IOL for a complete listing of indications, warnings and precautions.
REFERENCES:
- Clareon® PanOptix® Trifocal Hydrophobic Acrylic IOL Model CNWTT0 2021 Directions for Use.
- Lehmann, R., Maxwell, A., Lubeck, DM, Fong, R., Walters, TR, Fakadej, A. Effectiveness and Safety of the Clareon Monofocal Intraocular Lens: Outcomes from a 12-Month Single-Arm Clinical Study in a Large Sample. Clin Ophthalmol. 2021;15:1647-1657. Published 2021 Apr 20.
- Kohnen T, Herzog M, Hemkeppler E, et al. Visual Performance of a Quadrifocal (Trifocal) Intraocular Lens Following Removal of the Crystalline Lens. Am J Ophthalmol. 2017;184:52-62.
- Carson D, Xu Z, Alexander E, Choi M, Zhao Z, Hong X. Optical bench performance of 3 trifocal intraocular lenses. J Cataract Refract Surg. 2016;42(9):1361-1367.
- Alcon Data on File, 2014.
- Alcon Data on File, 2016.
- Alcon Data on File, 2014.

