Dry Eye
Systane Family of Products
Dry eye relief that provides fast hydration and tear evaporation protection.1-3

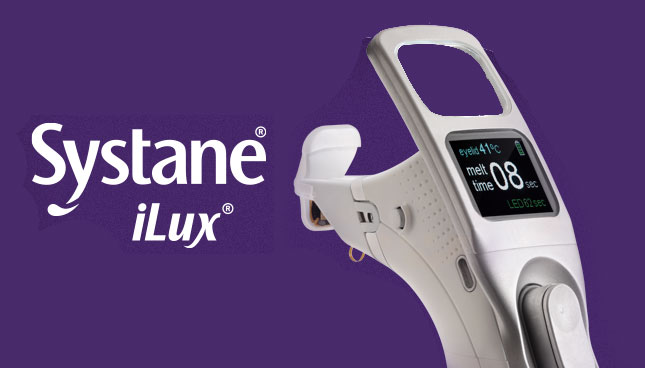
iLux MGD Procedure Platform
Personalized treatment to help address a major ‘root cause’ of evaporative dry eye
GENTEAL® TEARS
Fast relief and lasting protection against the burning and irritation that dry eye can cause.4
IMPORTANT PRODUCT INFORMATION
Systane® iLux2®
Indication: The Systane® iLux2® is indicated for the application of localized heat and pressure therapy in adult patients with meibomian gland dysfunction (MGD), which is associated with evaporative dry eye, and to capture/store digital images and video of the meibomian glands.
Contraindications:
Do NOT use the Systane® iLux2® in patients with the following conditions: Patients whose pupils have been pharmaceutically dilated; patients with ocular injury or trauma, chemical burns, or limbal stem cell deficiency (within prior 3 months); patients with active ocular herpes zoster or simplex of eye or eyelid or a history of these within prior 3 months; patients with cicatricial lid margin disease; patients with active ocular infection, active ocular inflammation or history of chronic, recurrent ocular inflammation within prior 3 months; patients with an ocular surface abnormality that may compromise corneal integrity; patients with lid surface abnormalities that affect lid function in either eye; patients with aphakia; or patients with permanent makeup or tattoos on their eyelids.
Warnings/Precautions:
Federal law restricts this device to sale by or on the order of a licensed healthcare practitioner.
The Disposable may not fit all eyes, such as eyes with small palpebral fornices. Use of the Systane® iLux2® is NOT recommended in patients with the following conditions: moderate to severe allergic, vernal or giant papillary conjunctivitis; severe eyelid inflammation; systemic disease conditions that cause dry eye; in patients who are taking medications known to cause dryness; patients with punctal plugs, or patients who have undergone ocular surgery within prior 3 months.
Potential Adverse Reactions:
Potential adverse effects may occur because of the procedure. These effects include but are not limited to, the onset or increase in: eyelid/eye pain requiring discontinuation of the treatment procedure, eyelid irritation or inflammation, temporary reddening of the skin, ocular surface irritation or inflammation (e.g., corneal abrasion, conjunctival edema or conjunctival injection (hyperemia)), and ocular symptoms (e.g., burning, stinging, tearing, itching, discharge, redness, foreign body sensation, visual disturbance, sensitivity to light).
Attention: Please refer to the User Manual for a complete list of contraindications, instructions for use, warnings and precautions for the Systane® iLux2®.
References
1. Korb D, Blackie C, Meadows D, Christensen M, Tudor M. Evaluation of extended tear stability by two emulsion based artificial tears. Poster presented at: 6th International Conference of Tear Film and Ocular Surface: Basic Science and Clinical Relevance; September 22-25, 2010; Florence, Italy.
2. Lane S, Paugh J, et al. An Evaluation of the in vivo Retention Time of a Novel Artificial Tear as Compared to a Placebo Control. Invest Ophthalmol Vis Sci. 2009;50(13):4679.
3. Torkildsen G. The effects of lubricant eye drops on visual function as measured by the Inter-blink interval Visual Acuity Decay test. Clin Ophtlamol. 2009;3:501-506.
4. Alcon Data on File, 2015
©2022 Alcon Inc. 4/22 US-DEOH-VLC-2200004


