
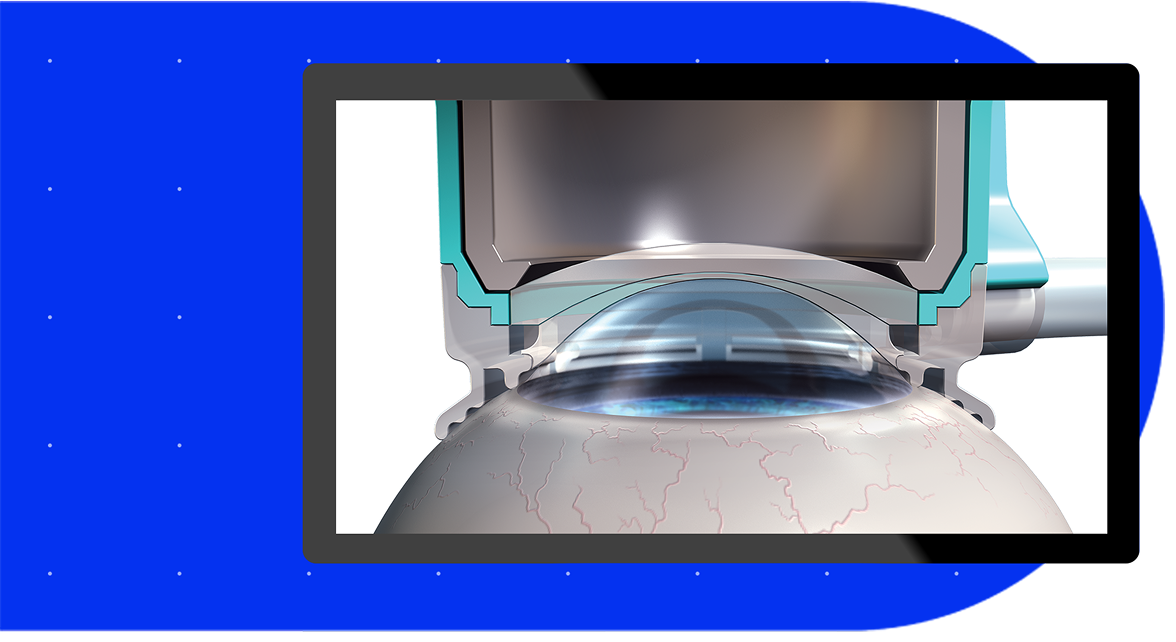
The LenSx® Laser with OnAxis Technology is a femtosecond laser system used in cataract surgery, offering a bladeless, precise and personalized approach that aims to improve accuracy at every step1.
Powered by innovation, LenSx® brings proven precision and efficiency2 with advanced astigmatism management features, such as capsular toric marking and the LaserArcs arcuate nomogram - offering a complete solution for astigmatism management.3
More surgeons trust LenSx® than any other platform4*
Proven Precision

Proven Efficiency
Proven Safety

Proven Outcomes
Proven Support
*Based on total number of procedures

Proven Precision
Bring more precision* for astigmatic patients at the time of cataract surgery5-7
Femtosecond laser assisted capsular toric marking provides an accurate reference to enable precise toric IOL alignment3,5,7
Capsular toric marks are permanent and can be used for verification of toric IOL rotational stability post-surgery3
*Versus manual marking. The mean postoperative toric IOL misalignment measured by the slitlamp was 2.4° + 1.96° for the digital-marking group and was 4.33° + 2.72° for the manual-marking group (P=0.003).
Reproducibility and accuracy at every step.8,9,10
Compared to manual procedures, LenSx® Laser delivers:

More precise, reproducible and reliable capsulotomies11,12


Delivers accurate, efficient and less damaging self-sealing cataract incisions in terms of its architecture, size and geometry13,14,15

Lower variability of anterior chamber depth (p<0.001) and better centration (p<0.001) compared to CCS16
The SoftFit® Patient Interface – Experience greater precision with the hydrogel lens insert of the LenSx® Laser.17

Proven Efficiency
Designed to improve operational efficiency by automating manual steps and streamlining the surgical workflow3

Advanced fragmentation patterns are designed to assist surgeons with their phaco techniques3

Connectivity to the ARGOS® Biometer with Image Guidance delivers more efficient astigmatism management3,5*


Customize your approach to each patient with versatile fragmentation. LenSx® empowers efficient treatment for a variety of lens densities18
*Compared to manual marking
Connectivity to the VERION Image Guided System delivers a new level of certainty.
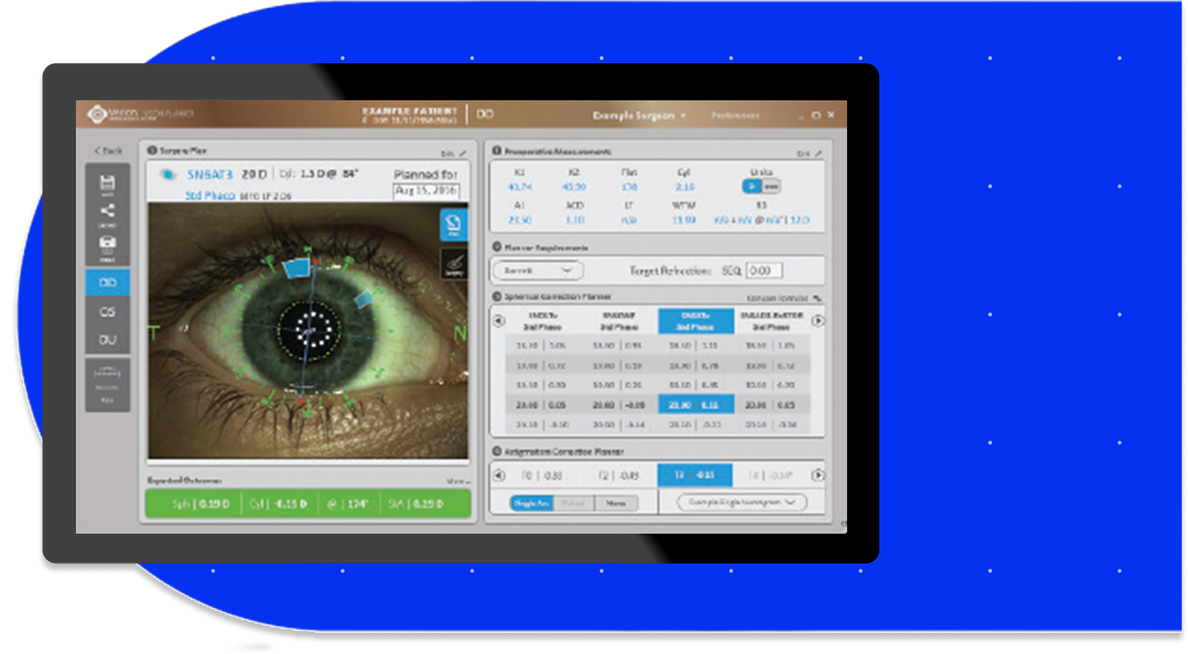
On average, laser cataract patients spend less time in the OR19*
Per patient, significant time savings were observed in the following categories19**
Overall, LenSx® was significantly faster than CATALYS^, with total time savings of 2.86 minutes.20†
*p<0.001; Roberts et al., (2018) conducted an RCT comparing CCS with a LenSx laser servicing two ORs in a hub-and-spoket model (N=299). The LenSx Laser was placed in the anesthetic room adjacent to the OR, and serviced patients feeding two ORs.
**Based on a real-world, prospective, observational, time-and-motion study with 89 patients undergoing cataract surgery across two surgery centers in the US.
†p<0.001.
^Trademarks are the property of their respective owners.

Proven Safety
The LenSx® laser shows proven safety with over 4.5 million* procedures and counting21

Advanced fragmentation patterns are designed to prioritize safety during the lens removal step3

LaserArcs has been trused in 200,000 procedures and is unique to LenSx® with OnAxis Technology22
*Alcon, Data on file. See important safety information.
Different sized and shaped fragmentation patterns, for a preferred
Phaco technique
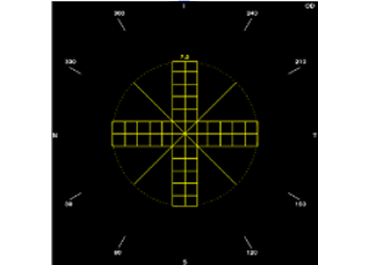

Thick cross with chop lines
Break a large percentage of the lens into smaller fragments, whilst chop lines retain some larger lens segments.
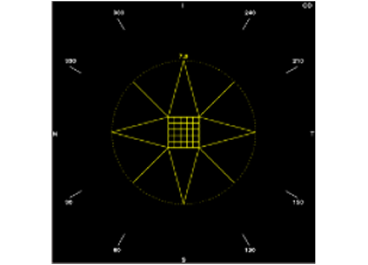

Star
Break the denser center part of the lens into smaller fragments.
Manage larger lens fragments away from the lens center with star pattern chop lines.
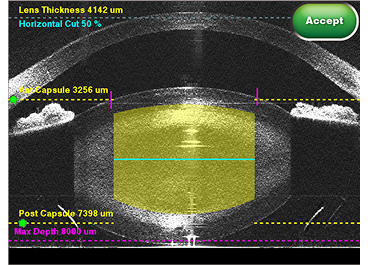

Horizontal cut
Create a cross-sectional cut in the lens, at a preferred depth and use chop lines to fragment.
The horizontal cut provides a positional reference in the lens during phaco.
Prioritize safety for complete reassurance


<2 minutes total time for patients under suction in cataract procedures23


<0.1% capsular tear rate with SoftFit® Patient Interface2
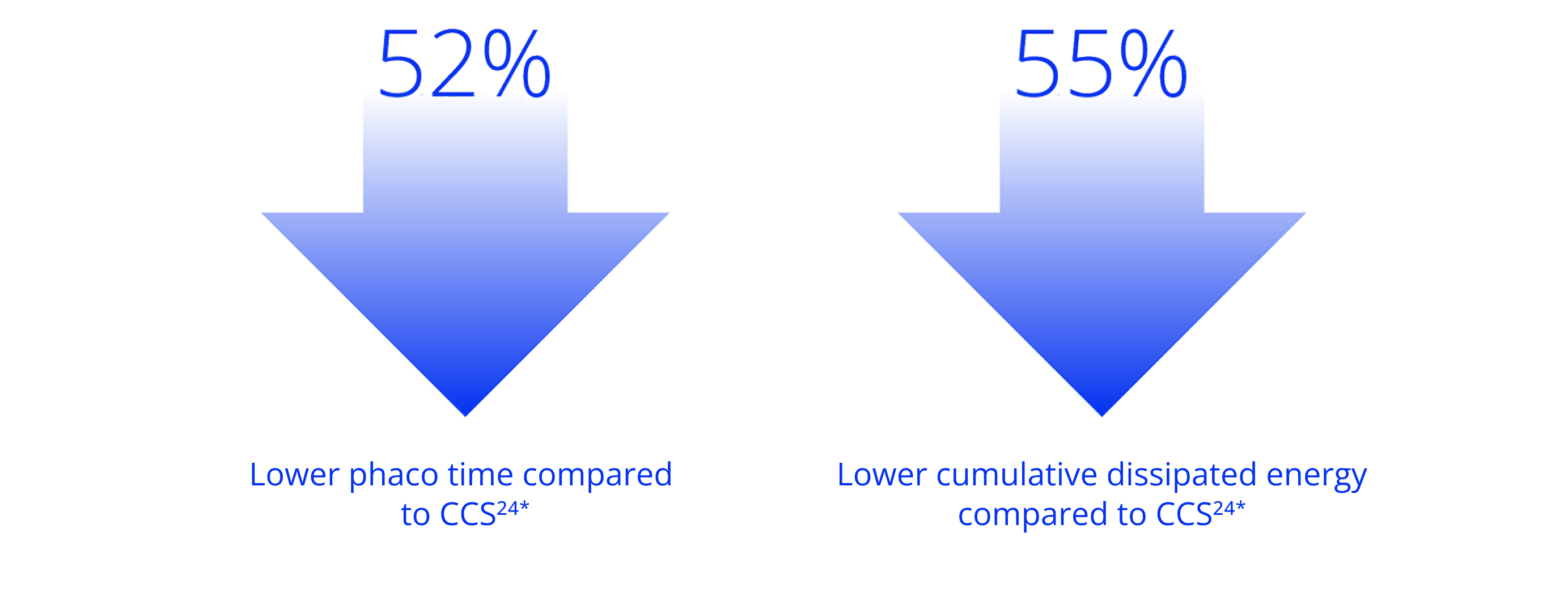
Based on 133 consecutive cases with 67 in the LenSx group; *p<0.001
- Decreased endothelial cell loss at 1 and 3 months versus conventional surgery8,10,25,26,27,28*
- Low rate of YAG capsulotomies compared to CCS29,30,31,32**
- Delivers quieter eyes than conventional surgery, by improving central corneal thickness at Day 1 and Week33,34
- Faster rehabilitation in hard cataracts, along with less phaco power and endothelial cell damage compared to CCS35
*p=0.05 for ECL and p=0.02 for CCT
**p<0.05 for overall complication rate, p=0.03 for PCR, p=0.04 for Hd:YAG capsulotomy, p<0.01 for PCO.


Proven Outcomes
Personalized astigmatism management options, designed to deliver excellent outcomes
94.2% of eyes fall within 0.5D of target refraction vs. 83.1% with manual36*


Shown to deliver a 50.7% reduction in mean post-op astigmatism vs. manual cases36**

Patients undergoing FLACS with arcuate incisions for reduction of astigmatism using the LaserArcs nomogram, postoperative UCVA was substantially similar to BCVA6
*Retrospective chart review of 231 patients for LenSx® and manual groups
**p<0.001. Retrospective chart review of 225 patients for LenSx® and manual groups

Proven Support
End to end integration, with service and support every step of the way


Technical Services
Improve reliability, peace of mind and drive business continuity


Clinical Services
Increase confidence, optimize performance and maximize user proficiency

Intelligent Services
Eliminate downtime, streamline workflow and transform performance
Alcon Experience Academy
For relevant training content from industry thought leaders
LenSx® Laser Important Product Information
CAUTION
Federal Law restricts this device to sale and use by or on the order of a physician or licensed eye care practitioner.
INDICATIONS FOR THE LENSX™ LASER (FOR ADULT PATIENTS):
In the creation of corneal cuts/incisions (single-plane, multiplane and arcuate) anterior capsulotomy and laser phacofragmentation during cataract surgery. Each of these procedures may be performed either individually or consecutively during the same surgery. In the creation of corneal cuts/incisions (single-plane, multi-plane, and arcuate) during Implantable Collamer Lens (ICL) surgery. In the creation of a corneal flap for patients undergoing LASIK surgery or other treatment requiring initial lamellar resection of the cornea. In the creation of corneal pockets for placement/insertion of a corneal inlay device; and for creation of corneal tunnels for the placement of corneal rings.
CONTRAINDICATIONS:
Conditions that would interfere with transmission of laser light at 1030 nm wavelength including presence of material or opacities anterior to or at laser plane. Examples include, but are not limited to: Corneal opacity, Corneal lesions, Corneal edema, Existing corneal implants, Blood or other material in the anterior chamber. Conditions that preclude safe applanation of the cornea, which include, but are not limited to: descemetocele with impending corneal rupture, existing corneal implants, hypotony, and glaucoma (glaucoma is not contraindicated with use of SoftFit® Patient Interface). Previous corneal incisions that might provide a potential space into which the gas produced by the procedure can escape. Residual, recurrent, active ocular or eyelid disease, including any corneal abnormality. Examples of corneal abnormality include, but are not limited to: corneal ectasia, recurrent corneal erosion, severe basement membrane disease. This device is contraindicated in pediatrics. Flap creation, tunnels, pockets, and cataract procedures cannot be combined into a single treatment. In addition to the above system contraindications, cataract surgery only contraindications include, but are not limited to: poorly dilating pupil, such that the iris is not at least 0.1 mm peripheral to the intended capsulotomy treatment at any point, i.e. the pupil diameter should be at least 0.2 mm larger than the capsulotomy diameter. Conditions which would cause inadequate clearance between any capsulotomy laser treatment and the corneal endothelium (applicable to capsulotomy only). Corneal thickness requirements that are beyond the range of the system. A history of lens instability or zonular dehiscence such that the lens is grossly decentered or unable to maintain its position during treatment. Any contraindications to cataract surgery.
WARNINGS: The LenSx™ Laser System should only be operated by a physician trained in its use. The LenSx™ Laser delivery system employs one sterile disposable Patient Interface consisting of an applanation lens and suction ring. Use of disposables other than those manufactured by Alcon may affect system performance and create potential hazards.
COMPLICATIONS: As with any surgical procedure, risk is involved. The following potential complications pose risks resulting from anterior capsulotomy, phacofragmentation, or creation of a partial thickness or full-thickness cut or incision as well as corneal procedures such as the creation of flap, tunnel or pocket: corneal edema, corneal decompensation, and subsequent severe loss of visual acuity, capsular tissue damage, corneal abrasion and epithelial defect, pain, electrical shock to non-patient and electrical shock to patient, bleeding, damage to ocular and non-ocular structures such as: iris damage due to trauma, physical injury to the patient and/or operator, corneal endothelial cell loss, and tissue damage not otherwise specified which includes complications during the procedure leading to incomplete or decentered flap and/or injury at the incision site posing a risk for wound leakage and subsequent inflammation and/or infection that could result in corneal opacity, Elevated IOP that may require additional intervention.
ATTENTION:
Refer to the LenSx™ Laser Operator’s Manual for a complete listing of indications, warnings and precautions.
References:
1. Slade S, Ignacio T, Spector S. Evaluation of a multifunctional femtosecond laser for the creation of laser in situ keratomileusis flaps. J Cataract Refract Surg. 2018;44:280-286.
2. Roberts TV et al. Update and clinical utility of the LenSx femtosecond laser in cataract surgery. Clin Ophthalmol. 2016;10:2021-2029.
3. Alcon Data on File, 2024. REF-25676.
4. Alcon Data on file, 2024. REF-24440.
5. Haeussler-Sinangin Y, Dahlhoff D, Schultz T, Dick HB. Clinical performance in continuous curvilinear capsulorhexis creation supported by a digital image guidance system. J Cataract Refract Surg. 2017;43(3):348–352.
6. Jones M, Hovanesian JA, Keyser A. Accuracy of the LaserArcs femtosecond cataract surgery arcuate incision nomogram in patients undergoing cataract surgery and astigmatism reduction. Clin Ophthalmol. 2023;17:681–689.
7. Elhofi AH, Helaly HA. Comparison Between Digital and Manual Marking for Toric Intraocular Lenses: A Randomized Trial. Medicine (Baltimore). 2015 Sep;94(38):e1618.
8. Popovic M, Campos-Moller X, Schlenker MB, Ahmed, II. Efficacy and Safety of Femtosecond Laser-Assisted Cataract Surgery Compared with Manual Cataract Surgery: A Meta-Analysis of 14 567 Eyes. Ophthalmology. 2016;123(10):2113-2126.
9. Qian DW, Guo HK, Jin SL, Zhang HY, Li YC. Femtosecond laser capsulotomy versus manual capsulotomy: a meta-analysis. Int J Ophthalmol. 2016;9(3):453-458.
10. Kolb CM, Shajari M, Mathys L, Herrmann E, et al. Comparison of femtosecond lase-assisted cataract surgery and conventional cataract surgery: a meta-analysis and systematic review. J Cataract Refract Surg. 2020;46:1075-1085.
11. Roberts TV, Lawless M, Sutton G, Hodge C. Anterior capsule integrity after femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2015;41(5):1109–1110.
12. Ranjini H, Murthy PR, Murthy GJ, Murthy VR. Femtosecond laser-assisted cataract surgery versus 2.2 mm clear corneal phacoemulsification. Indian J Ophthalmol. 2017;65(10):942-948.
13. Mastropasqua L, Toto L, Mastropasqua A, et al. Femtosecond laser versus manual clear corneal incision in cataract surgery. J Refract Surg. 2014;30(1):27-33.
14. Zhang X, et al. Performance of femtosecond laser-assisted cataract surgery in Chinese patients with cataract: A prospective, multicenter, registry study. BMC Ophthalmol. 2019;19:7.
15. Ferreira TB, Ribeiro FJ, Pinheiro J, et al. Comparison of surgically induced astigmatism and morphologic features resulting from femtosecond laser and manual clear corneal incisions for cataract surgery. J Refract Surg. 2018;34(5):322-329.
16. Toto L, Mastropasqua R, Mattei PA, Agnifili L, Mastropasqua A, Falconio G, Di Nicola M, Mastropasqua L. Postoperative IOL axial movements and refractive changes after femtosecond laser-assisted cataract surgery versus conventional phacoemulsification. J Refract Surg. 2015;31:524-530.
17. Bala C, Xia Y, Meades K. Electron Microscopy of Laser Capsulotomy Edge: Interplatform Comparison. J Cataract Refract Surg. 2014;40:1382-1389.
18. Shajari M, et al. Comparison of 2 laser fragmentation patterns used in femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2017;43:1571–1574.
19. Roberts HW, Wagh VB, Mullens IJM et al. Evaluation of a hub-and-spoke model for the delivery of femtosecond laser-assisted cataract surgery within the context of a large randomised controlled trial. Br J Ophthalmol 2018;102(11): 1556-1563.
20. Heit L, Datar M, Kyriakakos M, et al. Comparing the time-efficiency of two lasers used in femtosecond laser-assisted cataract surgery: a real-world observational study. J Cataract Refract Surg. 2023:10.1097/j.jcrs.0000000000001321.
21. Alcon data on file 2025. REF-27019.
22. Alcon Data on File, 2024. REF-24629.
23. Yeoh R. Practical differences between 3 femtosecond phaco laser platforms. J Cataract Refract Surg. 2014;40(3):510.
24. Kanellopoulos AJ, Asimellis G. Standard manual capsulorhexis/ultrasound phacoemulsification compared to femtosecond laser- assisted capsulorhexis and lens fragmentation in clear cornea small incision cataract surgery. Eye Vis. 2016;3:20.
25. Chen X, Chen K, He J, Yao K. Comparing the curative effects between femtosecond laser-assisted cataract surgery and conventional phacoemulsification surgery: a meta-analysis. PLoS One. 2016;11(3):e0152088.
26. Chen X, Xiao W, Ye S, Chen W, Liu Y. Efficacy and safety of femtosecond laser-assisted cataract surgery versus conventional phacoemulsification for cataract: a metaanalysis of randomized controlled trials. Sci Rep. 2015;5:13123.
27. Ye Z, Li Z, He S. A Meta-Analysis Comparing postoperative complications and outcomes of femtosecond laser-assisted cataract surgery versus conventional phacoemulsification for cataract. J Ophthalmol. 2017;3849152.
28. Al-Mohtaseb Z et al. Comparison of Corneal Endothelial Cell Loss Between Two Femtosecond Laser Platforms and Standard Phacoemulsification, J Refract Surg. 2017;33(10):708-712.
29. Chen M, Swinney C, Chen M. Comparing the intraoperative complication rate of femtosecond laser-assisted cataract surgery to traditional phacoemulsification. Int J Ophthalmol. 2015;8:201-203.
30. Roberts HW, Wagh VK, Sullivan DL, Hidzheva P, Detesan DI, Heemraz BS, Sparrow JM, O’Brart DPS. A randomized controlled trial comparing femtosecond laser-assisted cataract surgery versus conventional phacoemulsification surgery. J Cataract Refract Surg. 2019;45:11-20.
31. Tran DB, Vargas V, Potvin R. Neodymium: YAG capsulotomy rates associated with femtosecond laser-assisted versus manual cataract surgery. J Cataract Refract Surg. 2016;42:1470-1476.
32. Kovács I, Kránitz K, Sándor GL, Knorz MC, Donnenfeld ED, Nuijts RM, Nagy ZZ. The effect of femtosecond laser capsulotomy on the development of posterior capsule opacification. J Refract Surg. 2014;30:154-158.
33. Kohnen T, Mathys L, Petermann K, et al. Update on the comparison of femtosecond laser-assisted lens surgery to conventional cataract surgery: a systematic review and meta-analysis. Winter ESCRS Free Paper Presentation: Feb 2017.
34. Bouchet C et al. Comparing the efficacy, safety, and efficiency outcomes between lensx femtosecond laser-assisted cataract surgery and phacoemulsification cataract surgery: a meta-analysis. Value in Health. 2017;20(9):A800-A801.
35. Chen et al. Clinical outcomes of femtosecond laser–assisted cataract surgery versus conventional phacoemulsification surgery for hard nuclear cataracts. J Cataract Refract Surg 2017;43:486–491.
36. Yeu E, et al. A retrospective comparison of clinical outcomes associated with manual and femtosecond laser cataract surgery. Paper presented at: ASCRS; May 5-9, 2017; Los Angeles, CA.


