Wavelight plus
Introducing Alcon's next level
of Personalized Precision.1-3
Alcon's Next Generation Refractive Platform
Wavelight plus is Alcon's next generation in refractive surgery. Wavelight plus incorporates the evolution of the wavefront optimized pre-compensation algorithm by now adding compensation for non-symmetric aberrations, biomechanical changes and corneal healing as well as enhanced laser efficiency prediction.2,7
Measure
One device, multiple measurements1,4
Personalize
Ray tracing creates a personalized ablation profile1,2,5
Deliver
Go beyond the limits of 20/20 vision1,2,6
Measure
Evaluate the entire optical system. One Device. Multiple Measurements.1,2


Subjective refraction and K values
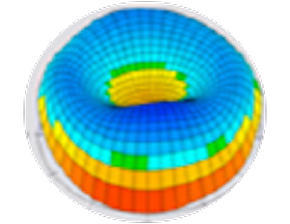

Wavefront
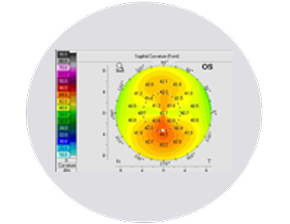

Anterior cornea
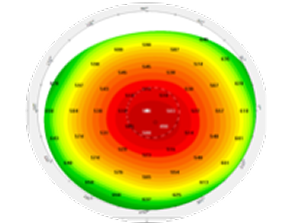

Pachymetry
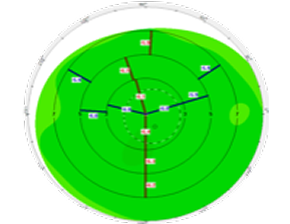

Posterior cornea
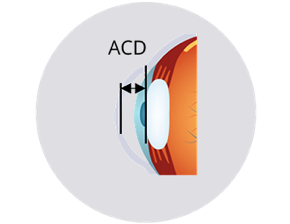

Anterior chamber depth and lens position
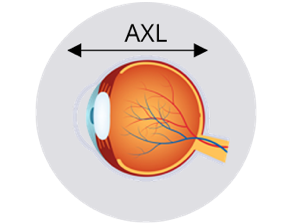

Axial length
Personalize
Ray Tracing for Individualized Ablation Profiles
Using a virtual 3D model of each individual eye, ray tracing technology delivers Alcon's most personalized procedure possible to each eye1
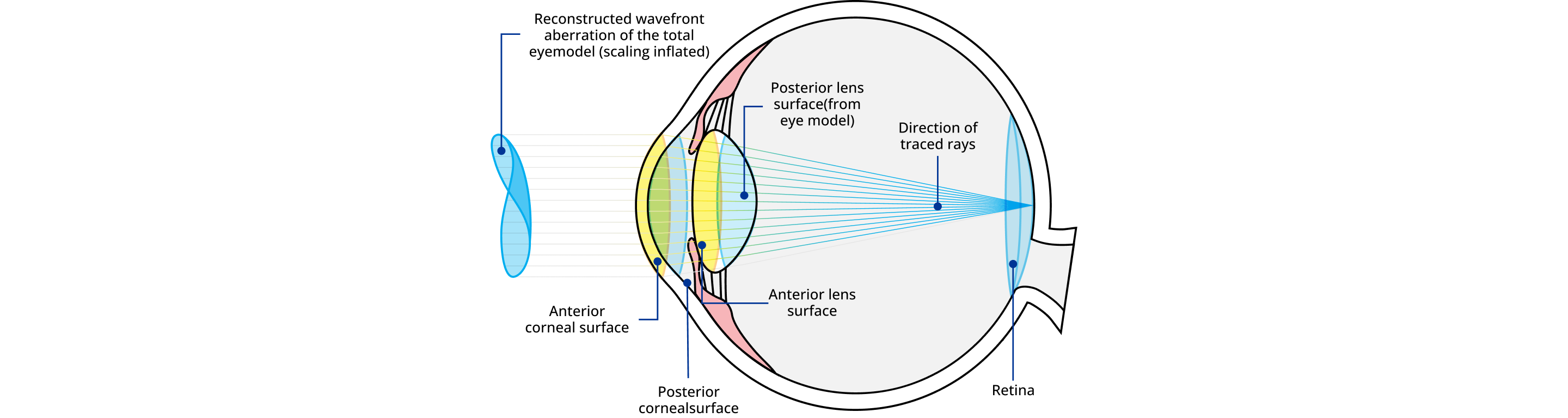
Utilizing the exact measurements, the Ray-Tracing calculation continues to iterate the model by recalculating and tracing rays until the optimal ablation treatment profile is achieved.1
Deliver
Unprecedented Patient Outcomes1,2,4
Delivering excellent visual outcomes, for whatever life might bring.1,2,4


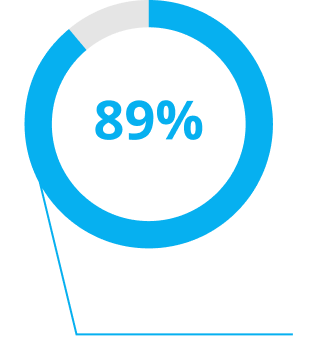

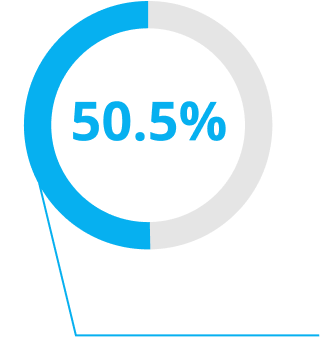

*In a real-world private practice setting-200 patients (400 eyes).
CAUTION: Federal law restricts this device to sale by or on the order of a physician.
DESCRIPTION AND CHARACTERISTICS:
The WaveLight® Plus laser systems is a non-contact ophthalmic diagnostic device designed to capture Scheimpflug images of the anterior segment of the eye, which includes the cornea, pupil, anterior chamber, and lens of the eye. Furthermore, it provides the axial dimensions of the eye using the technology of coherence interferometry. It can also measure the optical aberrations of the eye by applying Hartman-Shack wavefront technology
INDICATION:
The WaveLight® Plus laser systems is indicated for screening and diagnosis of adult patients who may undergo a customized photorefractive treatment with the WaveLight® Plus laser systems.
The WaveLight EX500 laser system in conjunction with WaveLight® Plus Sightmap is indicated for use in INNOVEYES Laser Assisted In-Situ Keratomileusis (“wavelight plus”1 LASIK) treatments:
- for the reduction or elimination of myopia or myopia with astigmatism, in eyes with spherical equivalent (SE) more than -1.00 and up to -9.00 diopters (D), with up to -8.00 D of spherical component (in minus cylinder format) and up to -3.00 D of astigmatic component at the spectacle plane, based on the INNOVEYES Sightmap Measured Refraction
- in patients with magnitude of the spherical equivalent (SE) difference between the Manifest Refraction (MRSE) and the Sightmap measured refraction SE being less than 0.75 D,
- in patients who are 18 years of age or older, and
- for patients with documentation of a stable manifest refraction defined as ≤ 0.5 D preoperative spherical equivalent shift over one year prior to surgery.
CONTRAINDICATIONS:
If you have any of the following situations or conditions, it is not recommended to have an examination with the WaveLight® Plus laser systems.
- Patients with open wounds and sores getting in contact with the head rest must not be examined.
There are no other known contraindications to the use of the WaveLight® Plus laser systems when used according to its approved indications.
TARGET PATIENT POPULATION:
The targeted patient population are patients which are selected for ophthalmic diagnosis consistent with the indications for use of the WaveLight® Plus laser systems.
INTENDED USERS:
The WaveLight® Plus laser systems may only be used by specially trained physicians, medical staff and optometrists who are well versed in its diagnostic abilities and possible dangers.
WARNINGS / PRECAUTIONS:
- Contact lens wearers must discontinue wearing hard or gas permeable lenses for at least 3 weeks and soft lenses for at least 1 week prior to examination.
- The examination takes place in a darkened room or with the help of a dark cloth covering the WaveLight® Plus laser systems and the patient’s head.
- The patient must be able to sit in an upright and comfortable position.
- The patient must be able to fixate steadily.
- Patients should not wear makeup at the day of examination.
- Avoid using eye-drops before examination. It may impact the diagnostic results and should be reported to the surgeon.
- Taking medication with influence on the hormonal balance can affect the consistency of the cornea.
- Results may be influenced by pregnancy and nursing. Hormonal changes can affect the consistency of the cornea.
- This device can cause flammable materials to ignite or explode
- Use of the controls or adjustments or performance procedures other than those specified in the user manual may result in hazardous radiation exposure.
MODE OF ACTION
The mode of action of the WaveLight® Plus laser systems is through the screening and diagnosis of anterior segment of the eye for planning custom refractive surgery treatments with the intent of improving vision.
STORAGE CONDITIONS
Store at -10-55°C (14-131°F).
ATTENTION: Refer to the Directions for Use labeling for a complete list of warnings, precautions, and adverse reactions.
Alcon Experience Academy
A non-promotional training and education resource for eye care professionals.
References:
1. Mrochen M, Bueler M, Donitzky C, Seiler T. Optical ray tracing for the calculation of optimized corneal ablation profiles in refractive treatment planning. J Refract Surg. 2008;24:S446-S451.
2. Kanellopoulos AJ, Maus M, Bala C, et al. International Multicenter, Myopic and Myopic Astigmatism Femto LASIK, Customized by Automated Ray-Tracing Ablation Profile Calculation: A Post market Study. Clin Ophthalmoly 2024;18:525-536.
3. He C, Bala C. Ray tracing guided myopic laser in situ keratomileusis - real world clinical outcomes. J Cataract Refract Surg. 2023;10-1097.
4. InnovEyes™ Sightmap Diagnostic Device User Manual 1089.
5. Kanellopoulos AJ. Initial outcomes with customized myopic LASIK, guided by automated ray tracing optimization: A novel technique. Clin Ophthalmol. 2020;14:3955–3963 2012;38(1):28-34. doi:10.1016/j.jcrs.2011.06.032.
6. Schumacher S, Seiler T, Cummings A, Maus M, Mrochen M. Optical ray tracing-guided laser in situ keratomileusis for moderate to high myopic astigmatism. J Cataract Refract Surg. 2012;38(1):28-34. doi:10.1016/j.jcrs.2011.06.032.
7. Bueeler M, & Mrochen M. Computer program for ophthalmologic surgery. (U.S. Patent No. US20080033408A1). 2008.
Please refer to relevant product’s Instructions for Use for complete list of indications, contraindications and warnings.


