AIR OPTIX® Plus HydraGlyde® for Astigmatism Contact Lenses Are Designed To Provide Comfortable, Clear Vision

Important safety information. Package insert. Fitting guide.
Give Your Astigmatic Patients Outstanding Comfort from Day 1 to Day 301-3

Comfort. Vision. Value.

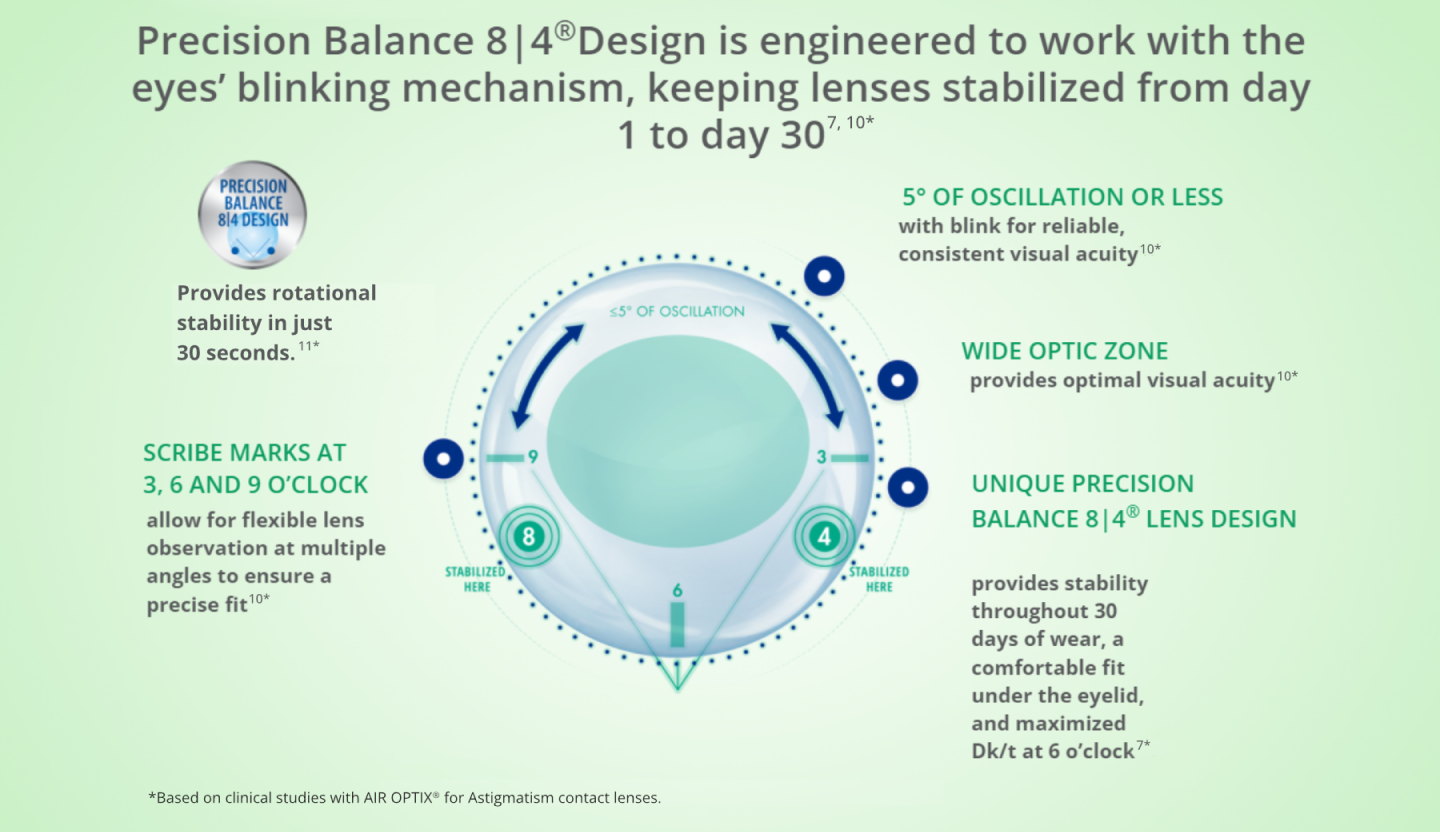
Dual Stability is The Key to Succesful Contact Lens Wear for Patients With Astigmatism
Tear Film Stability + On-Eye Stability


SmartShield® Technology creates an ultra-thin protective layer to shield lenses from irritating deposits all month long.4,5


PRECISION BALANCE 8|4® lens design is a modified prism-ballast design that has two anchor points for stabilization and provides excellent visual acuity all month. 6,7
SmartShield® Technology Helps Deliver Superior Overall Cholesterol Deposit Resistance Throughout the Lens.4,5,8,9
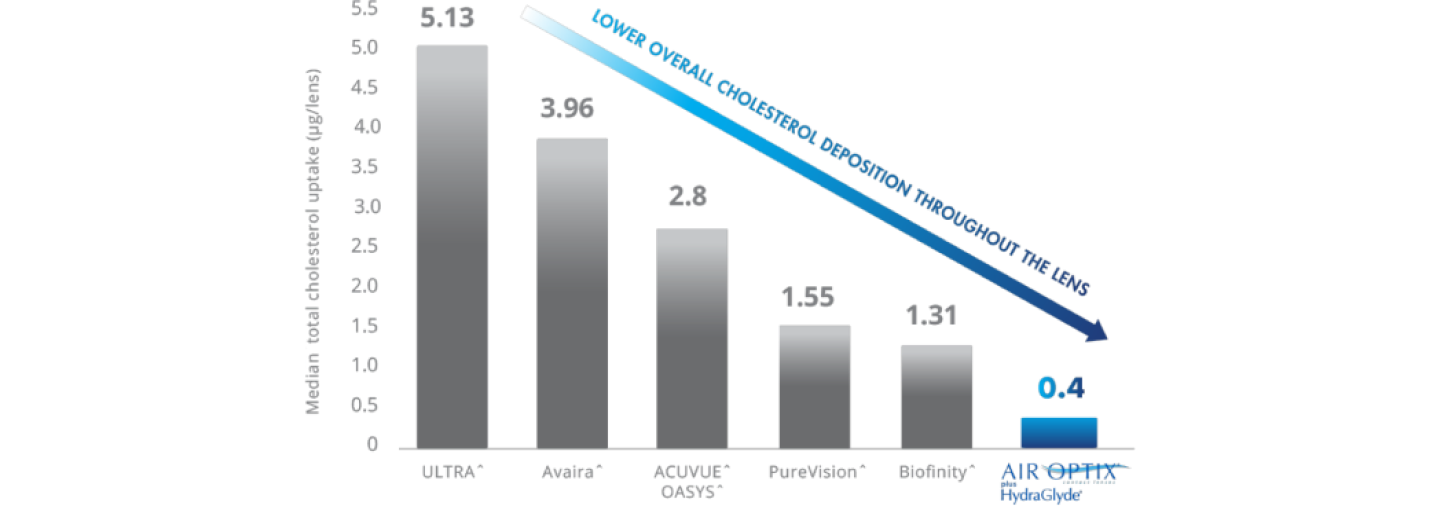
*Overall superior cholesterol deposit resistance of Air Optix® plus Hydraglyde® contact lenses, as compared to ACUVUE^ OASYS^ Lenses, PureVision^ lenses, Biofinity^ lenses and Avaira^ lenses, lenses worn daily for the manufacturer-recommended replacement period. CLEAR CARE® PLUS Cleaning & Disinfecting Solution used for cleaning and disinfection. all differences between AIR OPTIX® plus HydraGlyde® contact lenses and competitive brands statistically significant (p<0.05).
To Help Your Patients Get the Most From Their Contact Lenses, Recommend CLEAR CARE® PLUS and OPTI-FREE® Puremoist®

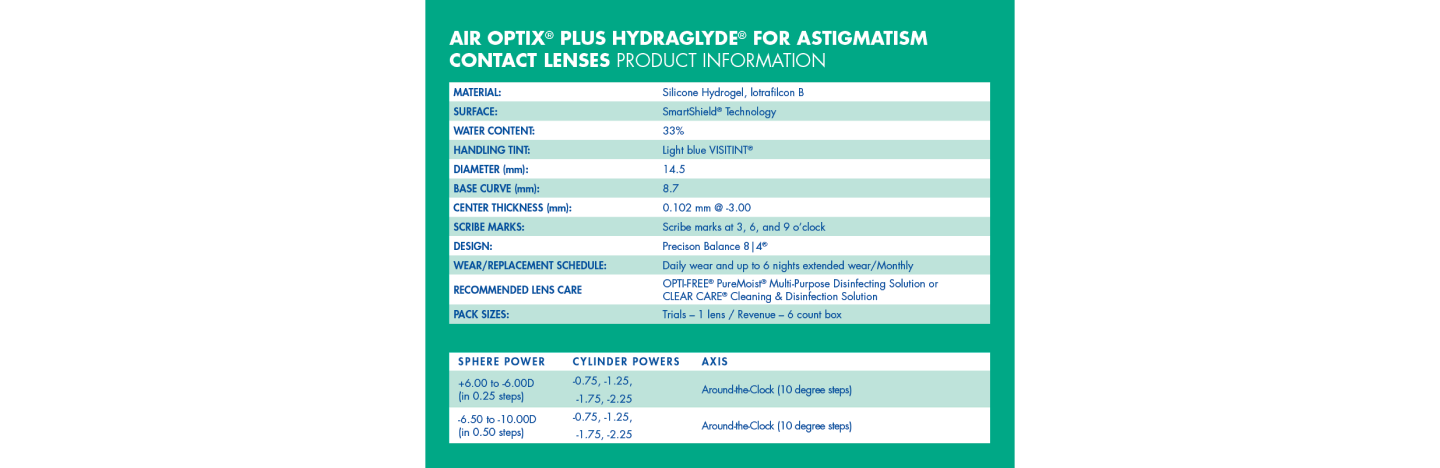
AIR OPTIX® plus HydraGlyde® for Astigmatism Contact Lenses Product Information


AIR OPTIX® plus HydraGlyde®
Give your patients outstanding comfort, from day 1 to day 30.1-3


AIR OPTIX® plus HydraGlyde® Multifocal
Designed to provide comfort and seamless vision at all distances, near through far.1,12
AIR OPTIX® plus Hydraglyde® for Astigmatism Product Inserts and Guidelines
AIR OPTIX® plus HydraGlyde® for Astigmatism Package Insert
AIR OPTIX® plus HydraGlyde® Patient Instruction Booklet
AIR OPTIX® plus HydraGlyde® Fitting Guide
See product instructions for complete wear, care and safety information. ![]()
Important information for AIR OPTIX® plus HydraGlyde® (lotrafilcon B) contact lenses:
For daily wear or extended wear up to 6 nights for near/far-sightedness. Risk of serious eye problems (i.e., corneal ulcer) is greater for extended wear. In rare cases, loss of vision may result. Side effects like discomfort, mild burning or stinging may occur.
Important information for AIR OPTIX® plus HydraGlyde® Astigmatism (lotrafilcon B) contact lenses:
For daily wear or extended wear up to 6 nights for near/far-sightedness, presbyopia and/or astigmatism. Risk of serious eye problems (i.e., corneal ulcer) is greater for extended wear. In rare cases, loss of vision may result. Side effects like discomfort, mild burning or stinging may occur.
Important information for AIR OPTIX® plus HydraGlyde® Multifocal (lotrafilcon B) contact lenses:
For daily wear or extended wear up to 6 nights for near/far-sightedness. Risk of serious eye problems (i.e., corneal ulcer) is greater for extended wear. In rare cases, loss of vision may result. Side effects like discomfort, mild burning or stinging may occur.
^Trademarks are the property of their respective owners.
- Eiden SB, Davis RL, Bergenske PD. Prospective study of lotrafilcon B lenses comparing 2 versus 4 weeks of wear for objective and subjective measures of health, comfort, and vision. Eye & Contact Lens. 2013;39(4):290-294.
- Based on a 30-day clinical study of 75 habitual lotrafilcon B lens wearers; Alcon data on file, 2017.
- Lemp J, Kern J. A comparison of real time and recall comfort assessments. Optom Vis Sci. 2016;93:Eabstract 165256.
- Nash W, Gabriel M, Mowrey Mckee M. A comparison of various silicone hydrogel lenses; lipid and protein deposition as a result of daily wear. Optom Vis Sci. 2010;87: E-abstract 105110.
- Nash W. Gabriel M. Ex vivo analysis of cholesterol deposition for commercially available silicone hydrogel contact lenses using a fluorometric enzymatic assay. Eye Contact Lens. 2014;40(5):277-282.
- In a randomized, subject-masked, multi-site clinical study with over 150 patients; Alcon data on file, 2005.
- Alcon data on file, 2005.
- Lemp J, Kern J. On-eye performance of lotrafilcon B lenses packaged with a substantive wetting agent. Poster presented at Optometry’s Meeting, the Annual Meeting of the American Optometric Association; June 21-25, 2017; Washington, D.C.
- Alcon data on file, 2016.
- In a randomized, subject-masked, multi-site clinical study with over 150 patients; Alcon data on file, 2005.
- In a randomized, subject-masked clinical study at 11 sites with 83 subjects; Alcon data on fille, 2006.
- Lemp J, Kern J. Alcon multifocal contact lenses for presbyopia correction. Paper presented at the Canadian Association of Optometrists Congress; June 28-30, 2017; Ottawa, ON.
