
This is CERTAINTY*
A gas-to-air mix you can trust from selection to injection.1-5
UNIFEYETM VIDEOS
MOA - Mechanism of Action
UNIFEYE(TM) vs Traditional Gas Mixing
How to Use

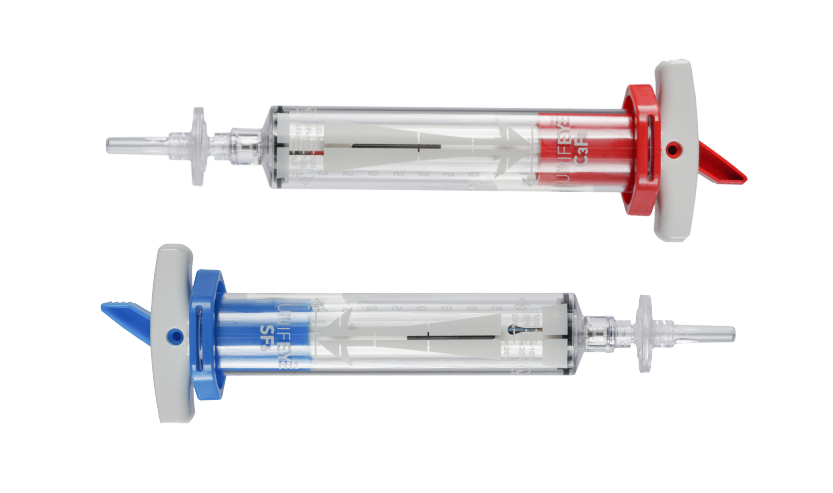
DESCRIPTION AND CHARACTERISTICS: The integrated UNIPURETM(C3F8 or SF6) Ophthalmic Gas pico-cylinder in the UNIFEYE and UNIPEXY Gas Delivery Systems contains undiluted, non-sterile, liquefied gas (perfluoropropane (C3F8) or sulfur hexafluoride (SF6)) under pressure. The gas is non-toxic, inert, non-flammable, odorless, and colorless.
The UNIFEYE Gas Delivery System is used to mix filtered UNIPURETM (C3F8 or SF6) Ophthalmic Gas with filtered air and inject the gas/air mix into the vitreous cavity of the eye. The prepared gas/air mixture for injection is sterile via the 0.2-micron filtration of the gas and air.
The UNIPEXY Gas Delivery System is used to inject filtered UNIPURETM (C3F8 or SF6) Ophthalmic Gas directly into the vitreous cavity to form a gas/air bubble. The prepared gas for injection is sterile via the 0.2-micron filtration of the gas.
INDICATION: The UNIPURETM (C3F8 or SF6) Ophthalmic Gas in the UNIFEYE and UNIPEXY Gas Delivery Systems is indicated for intraocular injection into the eye for the treatment of uncomplicated retinal detachments. Associated measures used include vitrectomy, fluid/air exchange, transconjunctival and transscleral cryotherapy, laser photocoagulation, and air/gas exchange.
CONTRAINDICATIONS: Proliferative vitreoretinopathy (PVR) greater than Stage C, the mental or physical inability to maintain the therapeutic position for 5 postoperative days, severe glaucoma with more than a minimum of vision field loss and a cup to disc ratio equal to or greater than 0.6; uveitis; severe peripheral retinal degeneration;
congenital malformations (such as coloboma), and any other condition that may facilitate the migration of the gas bubble out of the vitreous chamber; and high-altitude travel, including but not limited to airline travel.
• Air travel is contraindicated until the gas/air bubble has completely dissipated. Normal cabin pressure changes will cause a severe enlargement of the gas/air bubble with a resultant potential blinding, due to an increase in intraocular pressure (IOP)
• Patients should not travel through high elevations and over mountain ranges until the mixed gas/air bubble has dissipated.
WARNINGS / PRECAUTIONS: Use of Nitrous Oxide (N2O) must be stopped at least 15 minutes before injection of UNIPURETM (C3F8 or SF6) Ophthalmic Gas to ensure an adequate postoperative bubble is achieved. Do not administer N2O if a gas bubble is present.
Postoperative acute rises in IOP, which can threaten ocular blood flow lasting more than 10 minutes, may be controlled with pharmacologic methods, and/or paracentesis of aqueous fluid, and/or removal of some of the gas/air bubble. Patients with compromised ocular blood flow such as those with severe diabetic retinopathy or ocular ischemia are at greater risk of vascular occlusion following the use of a gas/air bubble.
Patient positioning following gas/air injection is of great importance. The gas/ air bubble must be properly situated with proper positioning to allow contact of the bubble against the targeted area of the retina.
Do not use and immediately notify Alcon if the UNIFEYE or UNIPEXY Gas Delivery System and/or the packaging are received in a defective condition.
After surgery, provide the items below to the patient:
• Wristband with insert
• Patient Implant Card
• Information brochure (located at www.ifu.alcon.com)
These take-home patient materials contain important information about the surgery and postoperative instructions.
UNIFEYE WARNING: Failure to properly complete each step as specified in the mixing procedure or interfering with gas delivery system activation may result in inaccurate gas/air mix ratios (higher or lower mix ratio than gas delivery system indicates).
UNIPEXY WARNING: Failure to properly complete each step as specified in the syringe filling procedure or interfering with gas delivery system activation may result in dilution of gas. Manual retraction of the Syringe Plunger prior to completion of syringe filling or after syringe filling will allow air to enter the syringe.
ADVERSE REACTIONS: Operative complications associated with the use of gas/air ocular endotamponades in eyes with or without vitrectomy may include; central retinal artery occlusion, subconjunctival gas, subretinal hemorrhage, small subretinal gas bubble, hypotony, choroidal hemorrhage, choroidal detachment, crystalline lens touch by needle, hyphema, escape of mixed gas/air through the surgical incisions, vitreous or iris incarceration at the wound, and elevated IOP, which may require additional medical or surgical intervention to reduce pressure.
ATTENTION: Refer to the Directions for Use labeling for a complete list of important safety information, instructions, warnings, precautions, and adverse reactions.
*Within 1.5% accuracy to all applicable accuracy claims
References
1. Alcon Data on file. 2018 (102-004-080).
2. Alcon Data on file. 2018 (102-004-081).
3. UNIFEYETM Directions for Use.
4. ISPAN® Directions for Use.
5. Alcon Surgical Retina Product Catalog. 2019; US-CON-1900001