AIR OPTIX™ plus HydraGlyde™ contact lenses combine 2 unique technologies into one outstanding lens

Give your patients outstanding comfort from day 1 to day 301-3*
*Based on a clinical study with AIR OPTIX® AQUA, AIR OPTIX® for Astigmatism, and AIR OPTIX® AQUA MULTIFOCAL Contact lenses.
†Based on a ProVoice Survey of ECPs from January 2019 - December 2019.
Comfort. Vision. Value.
Patients rated AIR OPTIX® plus HydraGlyde® a 9 out of 10 on initial insertion comfort4,5†
In a separate survey, 4x more patients preferred AIR OPTIX® plus HydraGlyde® over their previous two-week or monthly replacement lenses6*

*While using AOSEPT PLUS® or OPTI-FREE® PureMoist® for daily cleaning and disinfection.
†Based on average subjective rating on a scale of 1 (Poor) to 10 (Excellent) from a clinical trial of habitual AIR OPTIX AQUA contact lens wearers using AIR OPTIX plus HydraGlyde to determine on-eye performance (n=45)
SmartShield® Technology creates an ultra-thin protective layer to:
- Minimize the exposed silicone on the lens surface10
- Protect lenses from irritating deposits all month long7-9
- Protect from everyday use of cosmetics, hand creams and makeup removers12-14

SmartShield® Technology designed to help provide excellent overall cholesterol deposit resistance throughout the lens.4,7-9,19
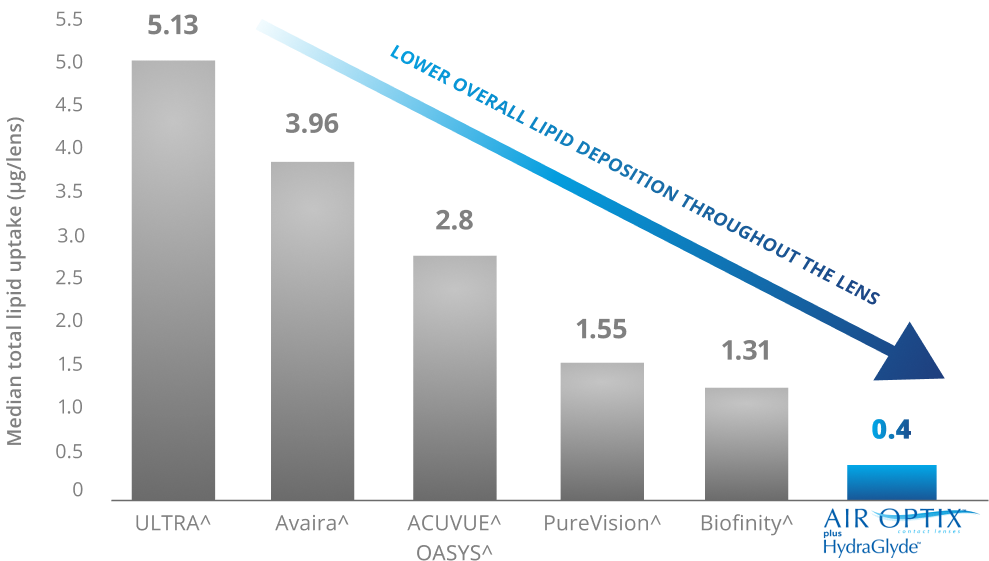
*Overall excellent cholesterol deposit resistance of AIR OPTIX™ plus Hydraglyde™ contact lenses, as compared to ACUVUE^ OASYS^ Lenses, PureVision^ lenses, Biofinity^ lenses and Avaira^ lenses, lenses worn daily for the manufacturer-recommended replacement period. AOSEPT PLUS® Cleaning & Disinfecting Solution used for cleaning and disinfection. All differences between AIR OPTIX™ plus Hydraglyde™ contact lenses and competitive brands statistically significant. (p<0.05)
^Trademarks are the property of their respective owners.
HydraGlyde™ Moisture Matrix Technology surrounds lenses in long-lasting surface moisture to help keep the lens surface hydrated16,17

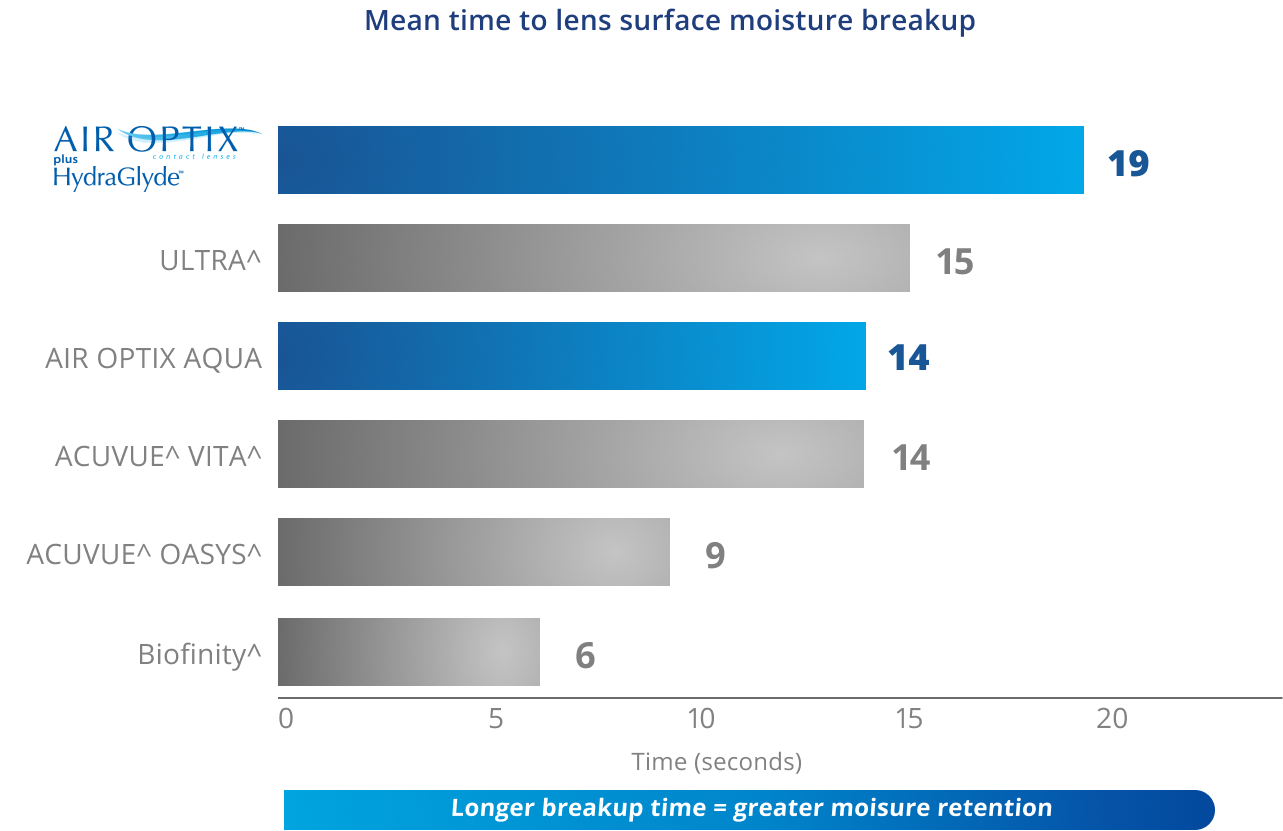
*Based on in vitro testing on sphere lenses soaked in phosphate-buffered saline solution for 16 hours; n=10 lenses analyzed per brand. + p<0.05 vs. all comparator lenses.
To help your patients get the most from their contact lenses, offer AOSEPT PLUS® with HydraGlyde™ and OPTI-FREE® PureMoist®

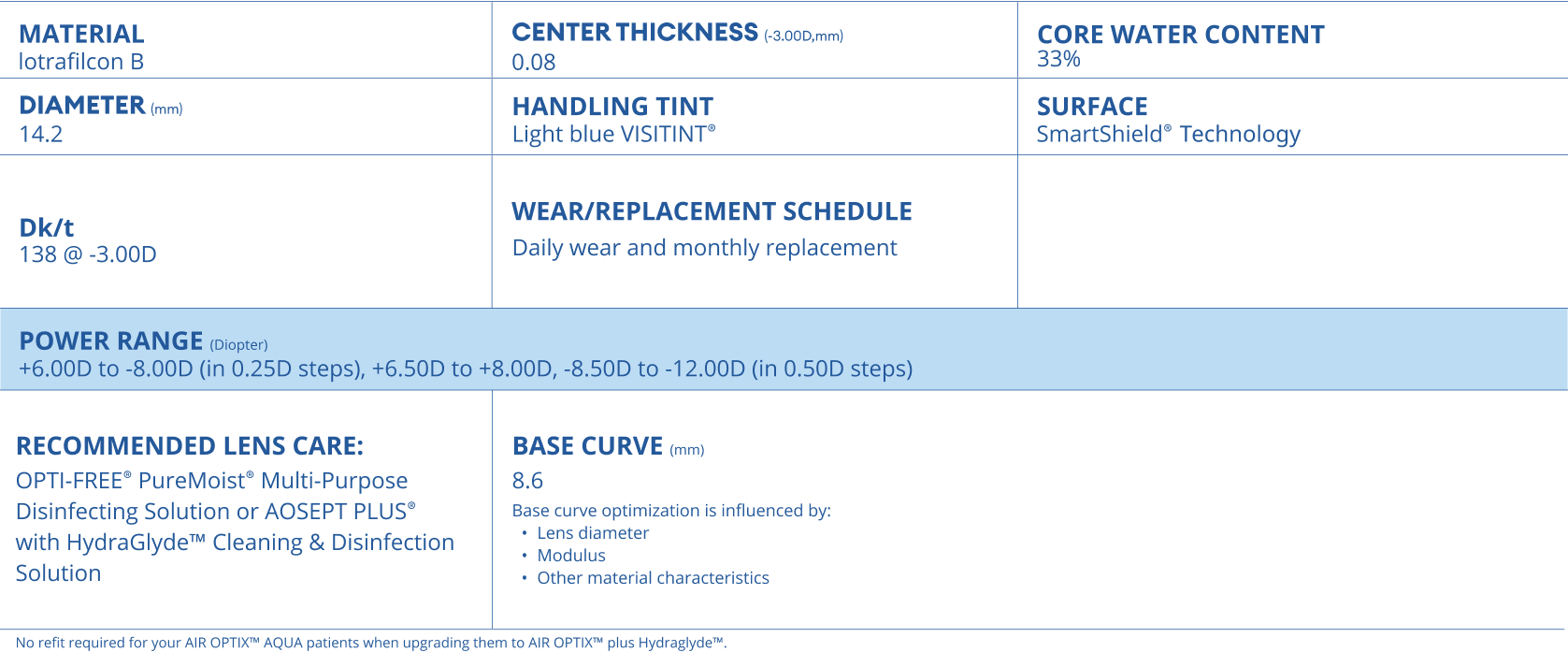
AIR OPTIX™ plus HydraGlyde™ contact lenses technical specification


AIR OPTIX™ plus HydraGlyde™ Multifocal
A multifocal contact lens designed to provide outstanding comfort from day 1 to day 30.1-3*


AIR OPTIX® plus HydraGlyde® for Astigmatism
Outstanding stability with a predictable fit, and outstanding comfort from day 1 to day 30 for your astigmatic patients.1-3,18*
*Based on a clinical study with AIR OPTIX® AQUA AIR OPTIX® for Astigmatism and AIR OPTIX® AQUA MULTIFOCAL Contact lenses.
Reference Links
^Trademarks are the property of their respective owners.
-
Eiden SB, Davis RL, Bergenske PD. Prospective study of lotrafilcon B lenses comparing 2 versus 4 weeks of wear for objective and subjective measures of health, comfort, and vision. Eye & Contact Lens. 2013;39(4):290-294.
-
Based on a 30-day clinical study of 75 habitual lotrafilcon B lens wearers; Alcon data on file, 2017.
-
Lemp J, Kern J. A comparison of real time and recall comfort assessments. Optom Vis Sci. 2016;93:Eabstract 165256.
-
Lemp J, Kern J. On-eye performance of lotrafilcon B lenses packaged with a substantive wetting agent. Poster presented at Optometry’s Meeting, the Annual Meeting of the American Optometric Association; June 21-25, 2017; Washington, D.C.
-
In a randomized, double-masked clinical study with 337 patients at 25 sites; CIBA VISION data on file, 2008.
-
Based on a survey in the US of 229 patients and 18 eye care professionals; Alcon data on file, 2017.
-
Nash W, Gabriel M, Movrewy Mckee M. A comparison of various silicone hydrogel lenses; lipid and protein deposition as a result of daily wear. Optom Vis Sci. 2010;87: E-abstract 105110.
-
Nash W. Gabriel M. Ex vivo analysis of cholesterol deposition for commercially available silicone hydrogel contact lenses using a fluorometric enzymatic assay. Eye Contact Lens. 2014;40(5):277-282.
-
Alcon data on file, 2015. In vitro biological deposition comparison of Air Optix. Available on request
-
Rex J, Perry S, Lemp J. Concentrations of Silicon at Silicone Hydrogel Contact Lens Surfaces. Poster presented at BCLA Clinical Conference; May 29-31, 2015; Liverpool, England.
-
Guillon M, Maissa C, Wong S, Patel K, Lemp J. Tear film dynamics over silicone hydrogel contact lenses with different lipid deposition profiles. Optom Vis Sci. 2014;91:E-abstract 145196.
-
Luensmann D, Yu M, Yang J, Srinivasan S, Jones L. Impact of Cosmetics on the Physical Dimension and Optical Performance of Silicone Hydrogel Contact Lenses. Eye & Contact Lens. 2015;4(4):218-227.
-
Srinivasan S, Otchere H, Yu M, Yang J, Luensmann D, Jones L. Impact of Cosmetics on the surface properties of silicone hydrogen contact lenses. Eye & Contact Lens. 2015;41(4):228-235.
-
Luensmann D, van Doorn K, May C, Srinivasan S, Jones L. Physical Dimension and Optical Assessment of Currently Marketed Silicone Hydrogel Contact Lenses After Exposure to Cosmetics. American Academy of Optometry, San Antonio, USA, 2018.
-
Alcon data on file, 2015. In vitro study over 16 hours to measure wetting substantivity. Available on request.
-
Muya L, Lemp J, Kern JR, Sentell KB, Lane J, Perry SS. Impact of packaging saline wetting agents on wetting substantivity and lubricity. Invest Opthalmol Vis Sci. 2016;57:E-abstract 1463.
-
Tucker R, Lemp J, Guillon M. In vitro and on eye wettability of lotrafilcon B lenses packaged with a substantive wetting agent. Invest Ophthalmol Vis Sci. 2017;58(8):3070.
-
Alcon data on file, 2005. P-242-C-007 1, 2 & 3 O2 OPTIX Toric DW US Comparative Trials. Available on request
-
Alcon data on file, 2008. Ex vivo total cholesterol & total protein analysis of commercially available silicone hydrogel lenses. Available on request
ALWAYS READ THE LABEL AND FOLLOW THE DIRECTIONS FOR USE.
All content on this website is for informational purposes only, always talk to your health professional regarding your eye health or medical conditions.
