AIR OPTIX™ NIGHT & DAY™ AQUA
A flexible lens-wearing experience designed for up to 30 nights of continuous wear1†

AIR OPTIX™ NIGHT & DAY™ AQUA can help patients with SmartShield® Technology and great breathability2*
AIR OPTIX™ NIGHT & DAY™ AQUA contact lenses are approved for up to 30 days and nights of continuous wear.1†


SmartShield® Technology
Delivers a protective layer of moisture to help shield lenses from irritating deposits3-5


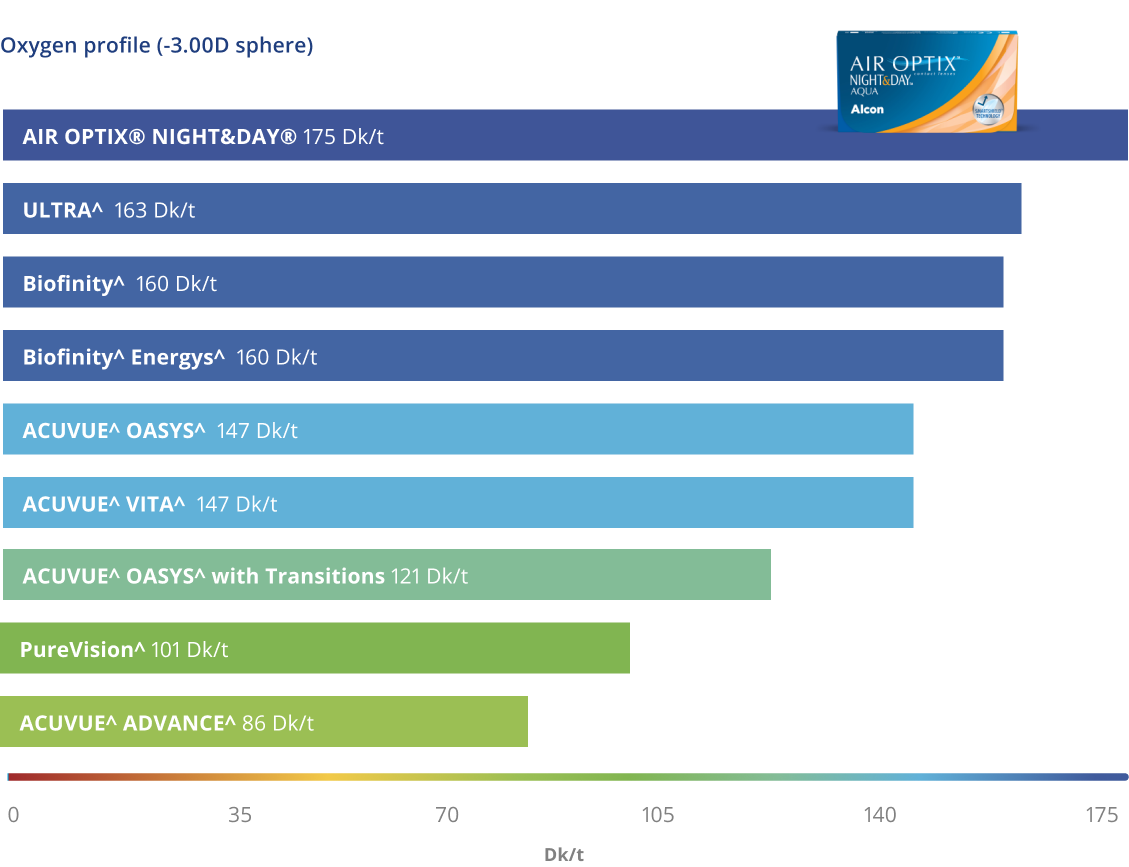
Highest Breathability2,6*
Oxygen continuously flows through the lens for white, healthy looking eyes2


A Winning Combination
For your patients who want to wake up to clear, comfortable vision.
*Dk/t = 175 @ -3.00D. Other factors may impact eye health.
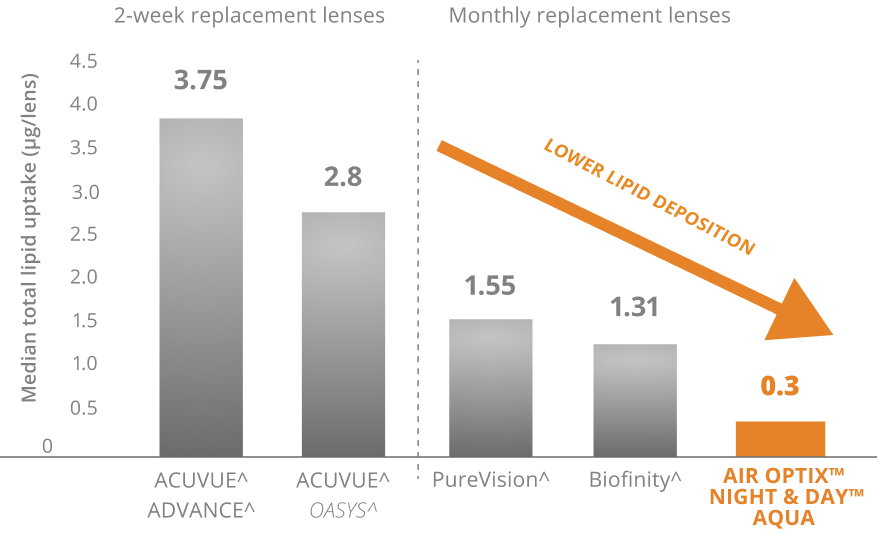
AIR OPTIX™ NIGHT & DAY™ AQUA offers overall deposit resistance and wettability that outperforms other lenses
Superior Lipid Deposit Resistance4,5
Lenses worn daily through manufacturer-recommended replacement period. AOSEPT™ PLUS with HydraGlyde™ Cleaning & Disinfecting Solution used for cleaning and disinfection. Median values represented.
The most breathable lens material on the market6
AIR OPTIX™ NIGHT & DAY™ AQUA is made from lotrafilcon A which was shown to have a low rate of microbial keratitis (MK)7
A rigorous post-market surveillance study of nearly 5,000 lotrafilcon A contact lens wearers for one year, and across 100+ clinical practices showed:
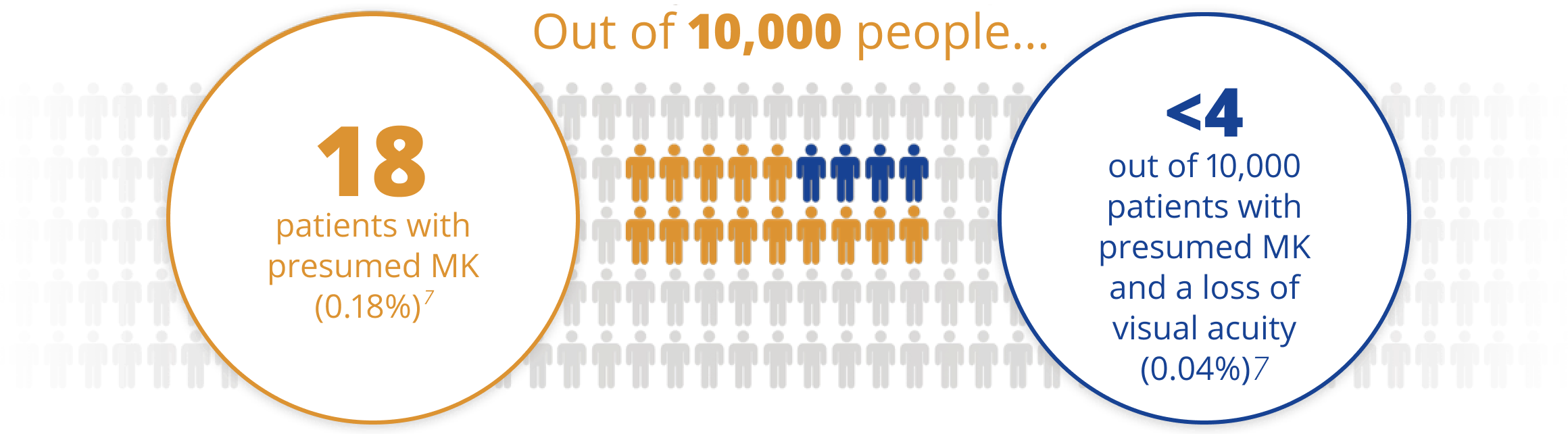
For those who were able to continuously wear the lenses ≥3 weeks, there was a lower rate of presumed MK than for those with <3 weeks of wear (p=0.02).
*Based on calucalted overall annual rate of MK
To help your patients get the most from their contact lenses, recommend AOSEPT™ PLUS with HydraGlyde™ or OPTI-FREE® Puremoist®

AIR OPTIX™ NIGHT & DAY™ AQUA Technical Specifications



AIR OPTIX™ plus HydraGlyde™ Multifocal
A multifocal contact lens designed to provide outstanding comfort from day 1 to day 30.8-10*


AIR OPTIX™ plus HydraGlyde™ for Astigmatism
Outstanding stability with a predictable fit, and outstanding comfort from day 1 to day 30 for your astigmatic patients.8-11*
*Based on clinical studies with AIR OPTIX™ AQUA lenses.
^Trademarks are the property of their respective owners
†Extended wear for up to 30 continuous nights, as prescribed by an eye care professional.
References:
1. AIR OPTIX™ NIGHT & DAY™ AQUA package insert.
2. Based on the ratio of lens oxygen transmissibilities; Alcon data on file, 2009, 2010.
3. Alcon data on file, 2008.
4. Nash W, Gabriel M, Mowrey-McKee M. A comparison of various silicone hydrogel lenses; lipid and protein deposition as a result of daily wear. Optom Vis Sci. 2010;87: E-abstract 105110.
5. Nash WL, Gabriel MM. Ex vivo analysis of cholesterol deposition for commercially available silicone hydrogel contact lenses using a fluorometric enzymatic assay. Eye Contact Lens. 2014;40(5):277-282.
6. Based on published manufacturer-provided DK/T values in Tylers Quarterly, 2021.
7. Schein O, McNally J, Katz J, Chalmers R. The incidence of microbial keratitis among wearers of a 30-day silicone hydrogel extended-wear contact lens. Ophthalmology. 2005;112(12):2172-79.
8. Eiden SB, Davis R, Bergenske P. Prospective study of lotrafilcon B lenses comparing 2 vrsus 4 weeks of wear for objective and subjective measures of health, comfort, and vision. Eye Contact Lens. 2013;39(4):290-294.
9. Lemp J, Kern J. A comparison of real time and recall comfort assessments. Optom Vis Sci. 2016;93:E-abstract 165256.
10. Based on a 30-day clinical study of 75 habitual lotrafilcon B lens wearers; Alcon data on file, 2017.
11. In a randomized, subject-masked, multi-site clinical study with over 150 patients; significance demonstrated at the 0.05 level, Alcon data on file, 2005.
See instructions for use for wear, care, precautions, warnings, contraindications and adverse effects.
