AutonoMe®
Delivery System
Control at every step1
AutonoMe® Delivery System
Control at every step1
AutonoMe® is the first and only automated, disposable, pre-loaded IOL delivery system.
Intuitive Control for a Smooth IOL Delivery1-3
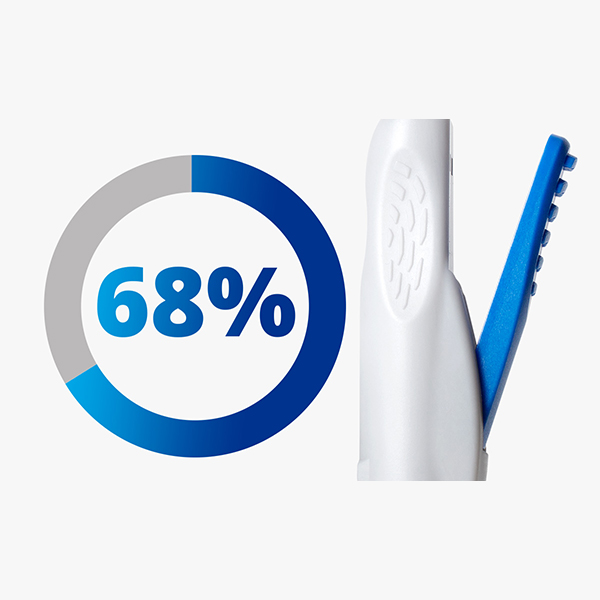

68% of surgeons found the delivery speed easy to control2*
The responsive speed control lever2
- Provides full control of plunger advancement
- Allows surgeons to precisely start, stop and linearly adjust speed at any time during surgery
- Helps surgeons focus more on the patient


70% of surgeons rated the overall experience of AutonoMe® as smooth and effortless2†
A unique delivery mechanism2,3
- Smooth, automated IOL delivery of up to 3 mm/sec
- Steady and continual motion providing a new level of control over IOL delivery speed
* Ease of controlling plunger advancement during IOL delivery; percent of total participants (n=136) from US, EU, Brazil & Japan. Study was conducted with porcine cadaver eyes (6 eyes per participant with over 800 eyes in total).
† Surgeons most commonly answered “Smooth” (43%) and “Effortless” (27%). Percent of total participants n=136 participants from US, EU, Brazil & Japan. Study was conducted with porcine cadaver eyes (6 eyes per participant with over 800 eyes in total).
Designed to fit comfortably in surgeons’ hands for intuitive single-handed control of the device1-3
- Designed for a natural fit to suit multiple surgical techniques, with no injection force required
- Single-handed design frees the second hand for globe stabilisation
- Precise control of nozzle tip orientation allows for consistent delivery of the IOL into the capsular
Designed to fit comfortably in surgeons’ hands for intuitive single-handed control of the device1-3
- Designed for a natural fit to suit multiple surgical techniques, with no injection force required
- Single-handed design frees the second hand for globe stabilisation
- Precise control of nozzle tip orientation allows for consistent delivery of the IOL into the capsular
Preserves Wound Integrity4*
AutonoMe® features a proprietary depth guard1,4
- Allows reduced stress and damage to the surrounding tissue
- Control of nozzle insertion depth
- Minimised incision enlargement
- Greater resistance to nozzle tip splitting
- Precise delivery into the capsular bag
*Ex-vivo, exploratory laboratory study performed on 32 human cadaver eyes.
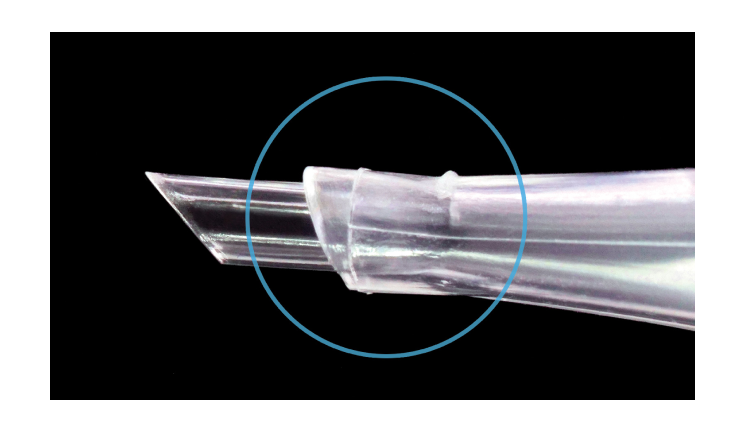
The AutonoMe® nozzle tip is designed to maintain wound integrity by5
- Preserving corneal tissue
- Minimising damage to Descemet’s membrane
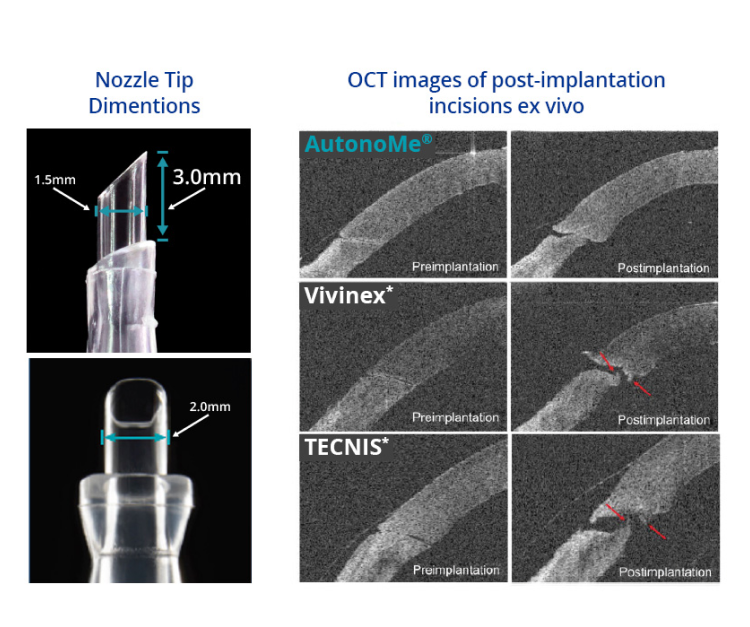
* Study conducted on human cadaver eyes that were randomly assigned to receive IOLs implanted with the preloaded delivery devices.
† Trademarks are the property of their respective owners
AutonoMe® demonstrates the lowest incidence of Descemet’s membrane detachment, compared to other delivery systems5
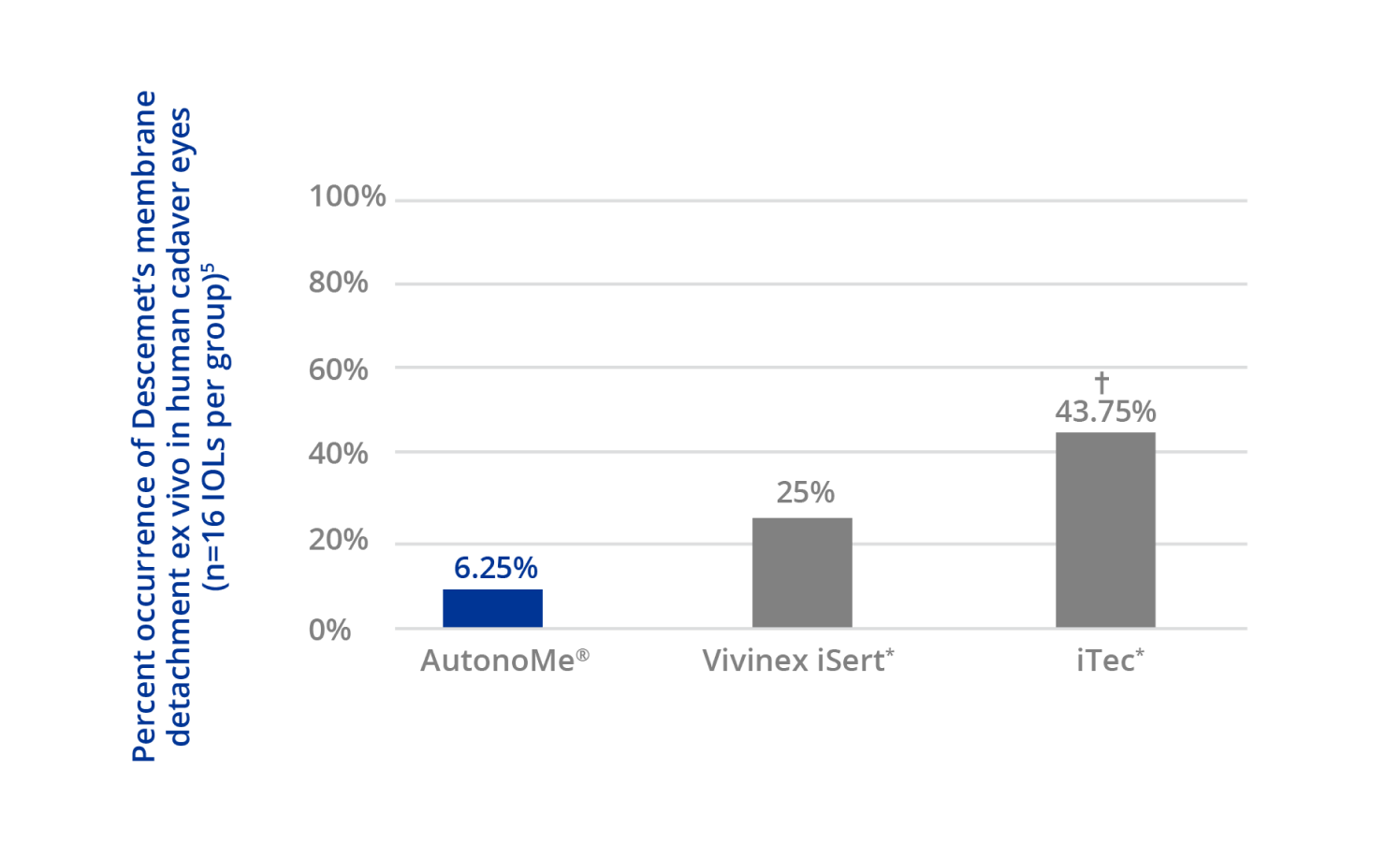
* Trademarks are the property of their respective owners.
† Denotes a statistically significant difference (p=0.037) from AutonoMe® using a Fisher’s exact test.
Easier to Use than Competitor Devices
AutonoMe® is reported to be easier to use compared to iTec* and iSert*2†
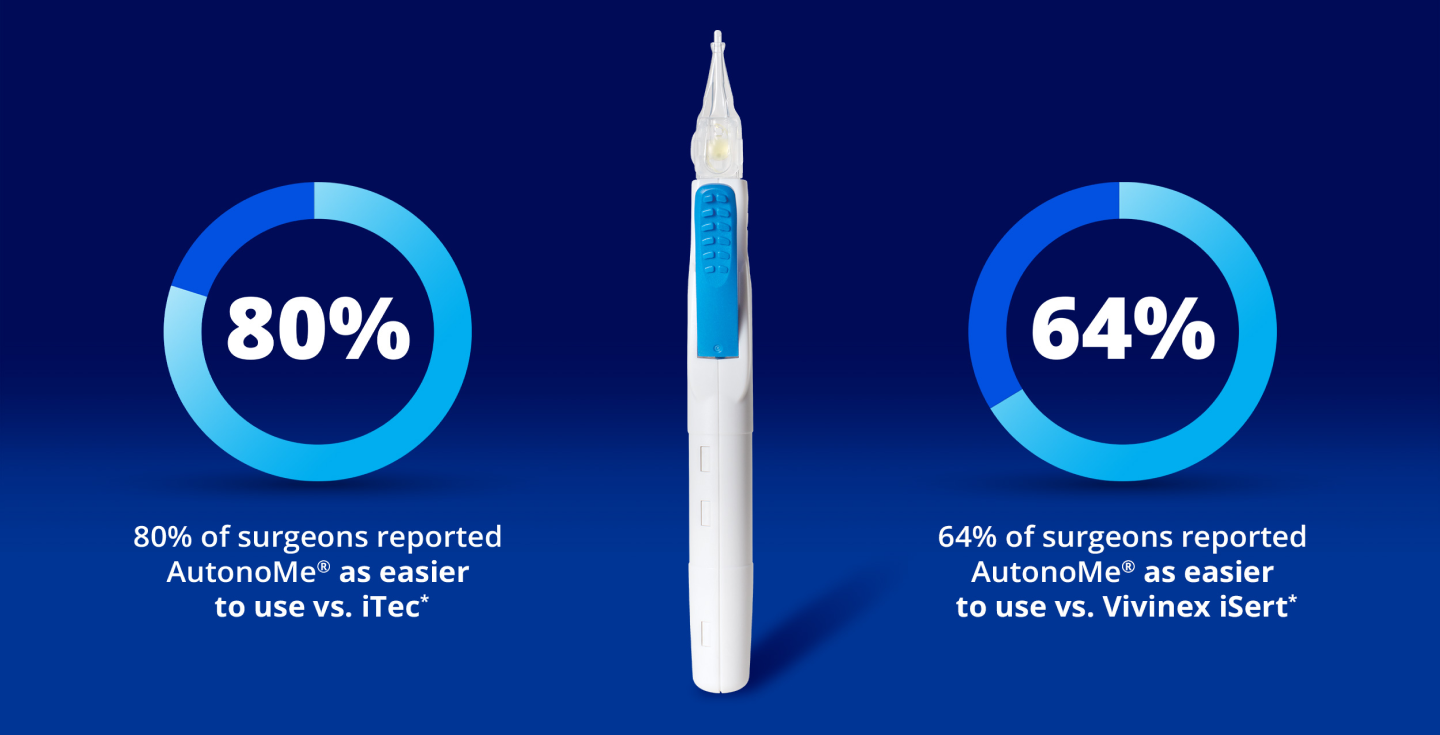
* Trademarks are the property of their respective owners.
† Porcine whole eye globes were used as the model in this study, as this model is extensively used for cataract surgery and IOL implantation studies.
Based on n = 17 for iTec and n = 8 for iSert.
AutonoMe® is ready to use in 3 simple steps1

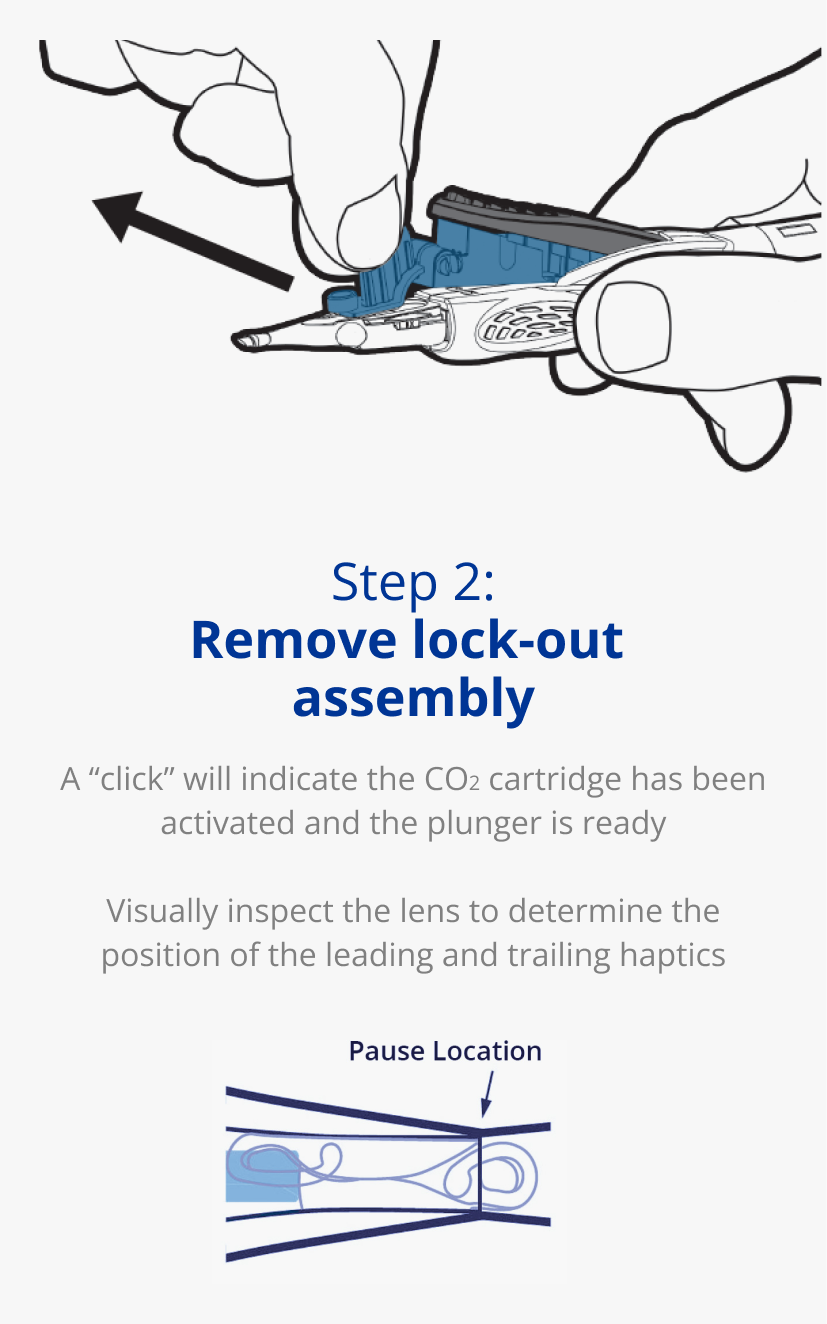
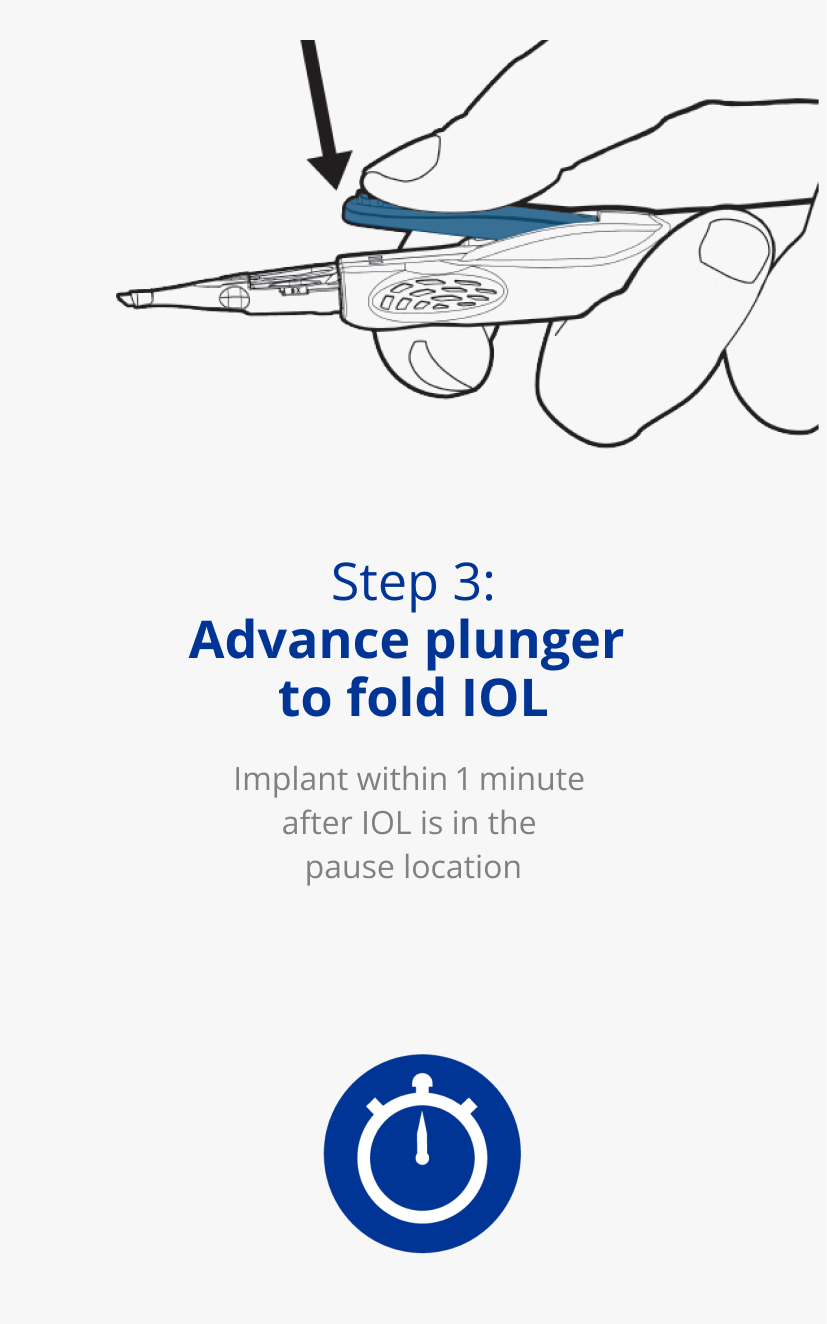
Learn how to use the
AutonoMe® Delivery System.
AutonoMe® is Pre-Loaded with
Clareon® IOL
Clareon® IOL offers outstanding refractive outcomes and unsurpassed clarity that lasts.6-14
AutonoMe® is Pre-Loaded with Clareon® IOL
Clareon® IOL offers outstanding refractive outcomes and unsurpassed clarity that lasts.6-14
AutonoMe Clinical Studies
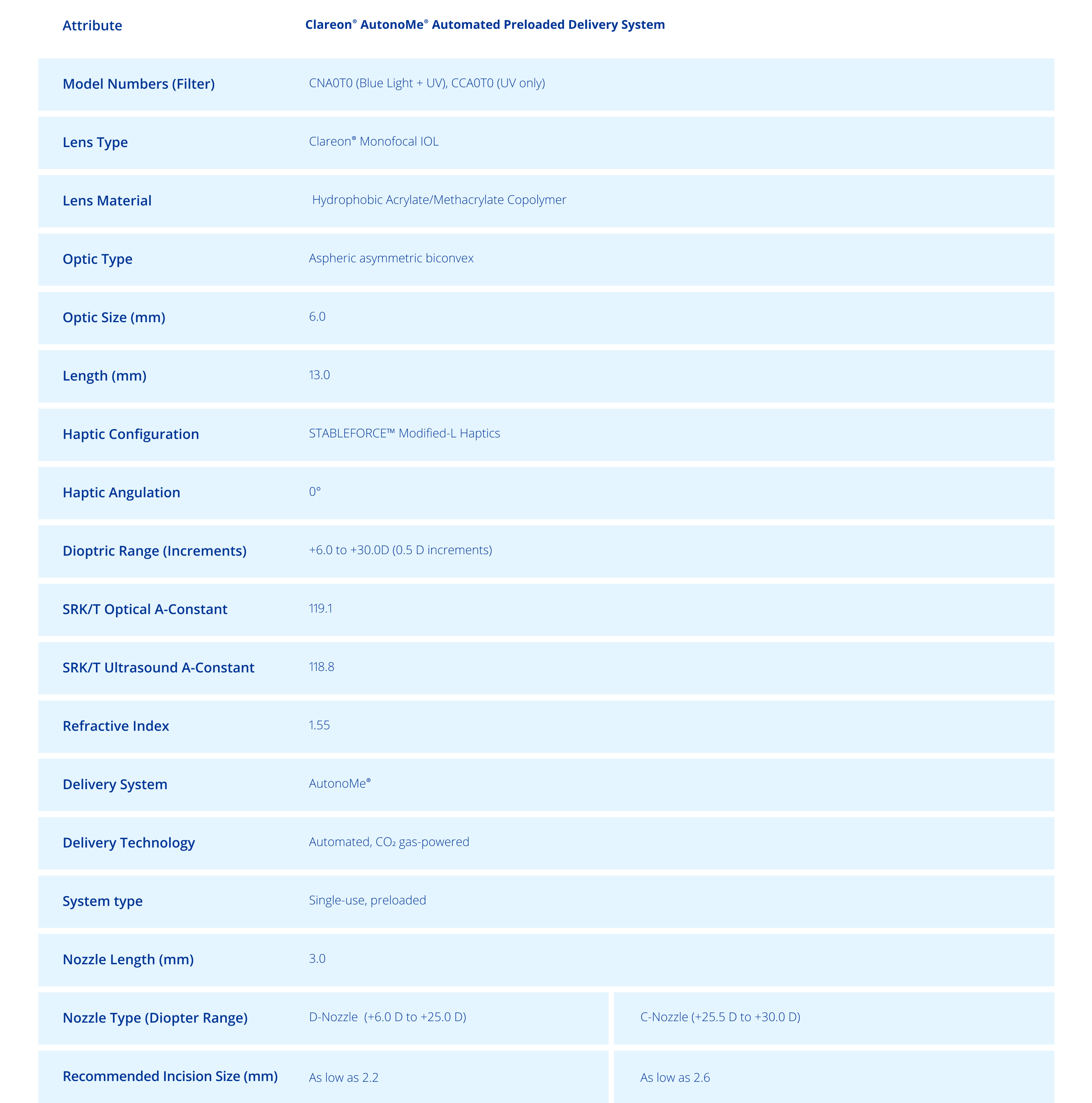
Technical Specifications1
Instructions for Use (IFU)
For a full list of indications, contraindications and warnings, please visit ifu.alcon.com and refer to the relevant product’s instructions for use.
Alcon Experience Academy
For relevant training content from industry thought leaders
1.Clareon® AutonoMe® Directions for Use.
2.Nozzle Preference and Delivery System Performance Study between AutonoMe and UltraSert V3.5 - TDOC 0053876
3.Verification Report, IOL Inserter Plunger Speed Testing. Alcon internal document REF-04194.
4.Liu J, Wolfe P, Hernandez V, Kohnen T. Comparative assessment of the corneal incision enlargement of 4 preloaded IOL delivery systems. J Cataract Refract Surg. 2020 Jul;46(7):1041-1046.
5.Alcon Data on File. TDOC-0054429.
6.Werner L, Thatthamla I, Ong M, et al. Evaluation of clarity characteristics in a new hydrophobic acrylic IOL. J Cataract Refract Surg. 2019;45:1490-1497.
7.Clareon® IOL Directions for Use.
8.Alcon Data on File, 2017. [TDOC-0050244]
9.Alcon Data on File, 2017. [TDOC-0054028]
10.Alcon Data on File, 2017. [TDOC-0053564]
11.Alcon Data on File, 2017. [TDOC-0053584]
12.Alcon Data on File, 2020. [TDOC-0057291]
13.Stanojcic N, O’Brart D, Hull C, et al. Visual and refractive outcomes and glistenings occurrence after implantation of 2 hydrophobic acrylic aspheric monofocal IOLs. J Cataract Refract Surg. 2020;46(7):986-994.
14.Oshika T, Fujita Y, Inamura M, Miyata K. Mid-term and long-term clinical assessments of a new 1-piece hydrophobic acrylic IOL with hydroxyethyl methacrylate. J Cataract Refract Surg. 2020;46(5):682-687.
Please refer to the relevant product direction for use for list of indications, contraindications and warnings. Find at https://ifu.alcon.com
©2022 Alcon Inc. 04/22 UKIE-CLA-2200001
