
WaveLight® Topolyzer® Vario Diagnostic Device
Precision measurements for personalized treatments


Topography, keratometry and pupillometry in a single device

Delivers complete map of the cornea for precise cyclotorsion control
Immediate visibility of visual defects with advanced, integrated mapping technology
Pupil centroid shift detection
Uses a placido disc system with 22,000 elevation points for ultimate precision

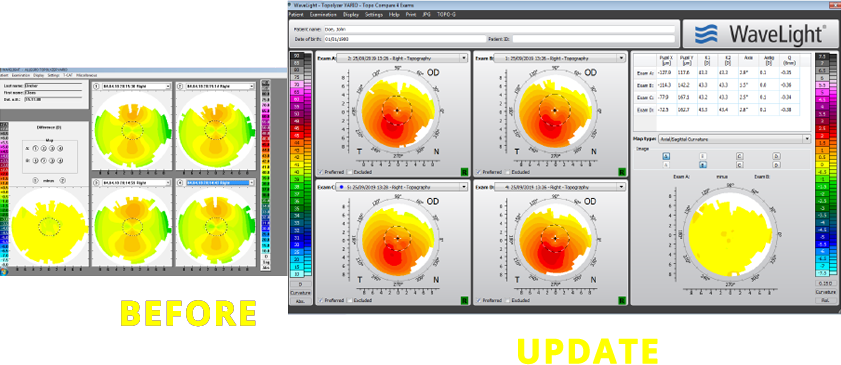
Navigate, view and compare corneal scans more efficiently-with fewer clicks
Keep it simple
- • Refined navigation system
- • Intuitive pupil detection and image selection
- • One-click printing
- • Simplified drop-down menus and shortcuts
- • WaveNet™ Integration
- • Smooth export functions
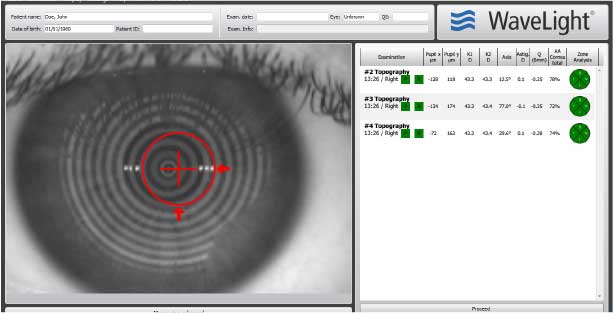
Get the whole picture
- • Scans cover more of the screen than the previous version
- • One click toggles between placido, topography and camera image views
- • Thumbnail previews improve organization
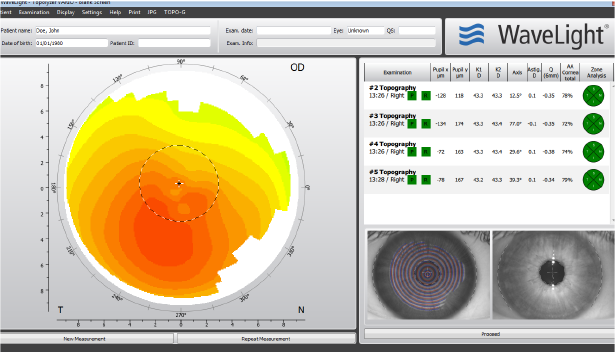
Easily compare scans
- • Exams are automatically ordered
- • Side-by-side positioning on screen
- • Switch from ‘preferred’ to ‘excluded’ with ease
Go Further with CONTOURA® Vision
Perform diagnostics with the advanced Vario platform, then treat with CONTOURA® Vision to provide a more personalized and satisfying experience to every one of your patients.
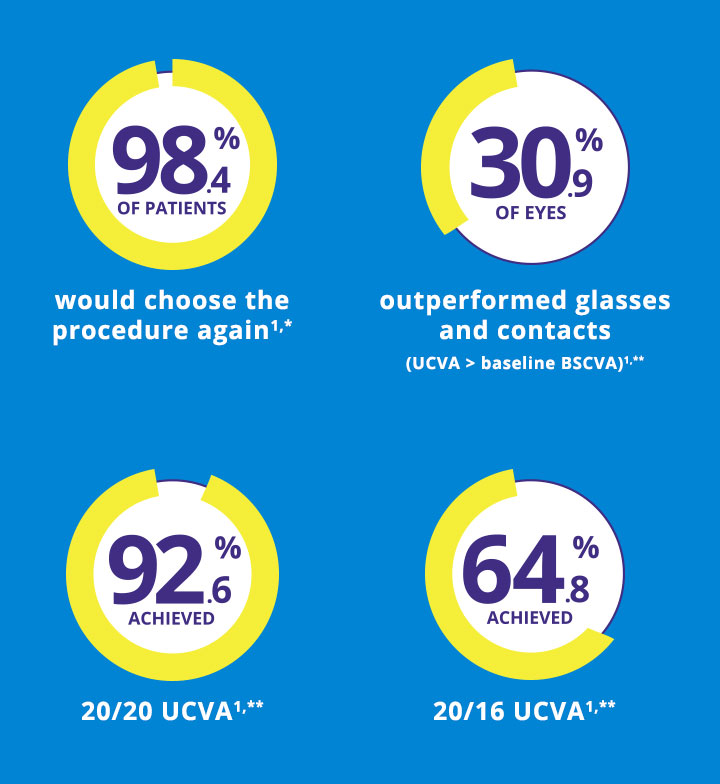
Surpass Your Patients'
Expectations1


5.2%
decrease in light sensitivity


8%
decrease in difficulty driving at night

4.8%
decrease in complaints of glare


8.7%
decrease in reading difficulty


3.2%
decrease in halos


2.8%
decrease in starbursts
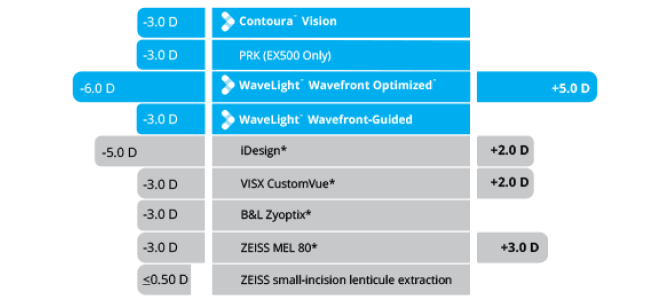
Sphere Treatments

Cylinder Treatments
WaveLight® Excimer Laser Systems Important Product Information
This information pertains to all WaveLight® Excimer Laser Systems, including the WaveLight® ALLEGRETTO WAVE®, the ALLEGRETTO WAVE® Eye-Q and the WaveLight® EX500. Caution: Federal (U.S.) law restricts the WaveLight® Excimer Laser Systems to sale by or on the order of a physician. Only practitioners who are experienced in the medical mangement and surgical treatment of the cornea, who have been trained in laser refractive surgery (including laser calibration and operation) should use a WaveLight® Excimer Laser System. Indications: FDA has approved the WaveLight® Excimer Laser systems for use in laser-assisted in situ keratomileusis (LASIK) treatments for: the reduction or elimination of myopia of up to - 12.00 D and up to 6.00 D of astigmatism at the spectacle plane; the reduction or elimination of hyperopia up to + 6.00 D with and without astigmatic refractive errors up to 5.00 D at the spectacle plane, with a maximum manifest refraction spherical equivalent of + 6.00 D; • the reduction or elimination of naturally occurring mixed astigmatism of up to 6.00 D at the spectacle plane; and the wavefront-guided reduction or elimination of myopia of up to -7.00 D and up to 3.00 D of astigmatism at the spectacle plane. In addition, FDA has approved the WaveLight® ALLEGRETTO WAVE® Eye-Q Excimer Laser System, when used with the WaveLight® ALLEGRO Topolyzer® and topography-guided treatment planning software for topography-guided LASIK treatments for the reduction or elimination of up to -9.00 D of myopia, or for the reduction or elimination of myopia with astigmatism, with up to -8.00 D of myopia and up to 3.00 D of astigmatism. The WaveLight® Excimer Laser Systems are only indicated for use in patients who are 18 years of age or older (21 years of age or older for mixed astigmatism) with documentation of a stable manifest refraction defined as ≤ 0.50 D of preoperative spherical equivalent shift over one year prior to surgery, exclusive of changes due to unmasking latent hyperopia. Contraindications: The WaveLight® Excimer Laser Systems are contraindicated for use with patients who: are pregnant or nursing; have a diagnosed collagen vascular, autoimmune or immunodeficiency disease; have been diagnosed keratoconus or if there are any clinical pictures suggestive of keratoconus; are taking isotretinoin (Accutane*) and/or amiodarone hydrochloride (Cordarone*); have severe dry eye; have corneas too thin for LASIK; have recurrent corneal erosion; have advanced glaucoma; or have uncontrolled diabetes. Warnings: The WaveLight® Excimer Laser Systems are not recommended for use with patients who have: systemic diseases likely to affect wound healing, such as connective tissue disease, insulin dependent diabetes, severe atopic disease or an immunocompromised status; a history of Herpes simplex or Herpes zoster keratitis; significant dry eye that is unresponsive to treatment; severe allergies; a history of glaucoma; an unreliable preoperative wavefront examination that precludes wavefront-guided treatment; or a poor quality preoperative topography map that precludes topography-guided LASIK treatment. The wavefront-guided LASIK procedure requires accurate and reliable data from the wavefront examination. Every step of every wavefront measurement that may be used as the basis for a wavefront-guided LASIK procedure must be validated by the user. Inaccurate or unreliable data from the wavefront examination will lead to an inaccurate treatment. Topography-guided LASIK requires preoperative topography maps of sufficient quality to use for planning a topography-guided LASIK treatment. Poor quality topography maps may affect the accuracy of the topography-guided LASIK treatment and may result in poor vision after topography-guided LASIK. Precautions: The safety and effectiveness of the WaveLight® Excimer Laser Systems have not been established for patients with: progressive myopia, hyperopia, astigmatism and/or mixed astigmatism, ocular disease, previous corneal or intraocular surgery, or trauma in the ablation zone; corneal abnormalities including, but not limited to, scars, irregular astigmatism and corneal warpage; residual corneal thickness after ablation of less than 250 microns due to the increased risk for corneal ectasia; pupil size below 7.0 mm after mydriatics where applied for wavefront-guided ablation planning; history of glaucoma or ocular hypertension of > 23 mmHg; taking the medications sumatriptan succinate (Imitrex*); corneal, lens and/or vitreous opacities including, but not limited to cataract; iris problems including , but not limited to, coloboma and previous iris surgery compromising proper eye tracking; or taking medications likely to affect wound healing including (but not limited to) antimetabolites. In addition, safety and effectiveness of the WaveLight® Excimer Laser Systems have not been established for: treatments with an optical zone < 6.0 mm or > 6.5 mm in diameter, or an ablation zone > 9.0 mm in diameter; or wavefront-guided treatment targets different from emmetropia (plano) in which the wavefront calculated defocus (spherical term) has been adjusted; In the WaveLight® Excimer Laser System clinical studies, there were few subjects with cylinder amounts > 4 D and ≤ 6 D. Not all complications, adverse events, and levels of effectiveness may have been determined for this population. Pupil sizes should be evaluated under mesopic illumination conditions. Effects of treatment on vision under poor illumination cannot be predicted prior to surgery. Adverse Events and Complications Myopia: In the myopia clinical study, 0.2% (2/876) of the eyes had a lost, misplaced, or misaligned flap reported at the 1 month examination. The following complications were reported 6 months after LASIK: 0.9% (7/818) had ghosting or double images in the operative eye; 0.1% (1/818) of the eyes had a corneal epithelial defect. Hyperopia: In the hyperopia clinical study, 0.4% (1/276) of the eyes had a retinal detachment or retinal vascular accident reported at the 3 month examination. The following complications were reported 6 months after LASIK: 0.8% (2/262) of the eyes had a corneal epithelial defect and 0.8% (2/262) had any epithelium in the interface. Mixed Astigmatism: In the mixed astigmatism clinical study, two adverse events were reported. The first event involved a patient who postoperatively was subject to blunt trauma to the treatment eye 6 days after surgery. The patient was found to have an intact globe with no rupture, inflammation or any dislodgement of the flap. UCVA was decreased due to this event. The second event involved the treatment of an incorrect axis of astigmatism. The axis was treated at 60 degrees instead of 160 degrees. The following complications were reported 6 months after LASIK: 1.8% (2/111) of the eyes had ghosting or double images in the operative eye. Wavefront-Guided Myopia: The wavefront-guided myopia clinical study included 374 eyes treated; 188 with wavefront-guided LASIK (Study Cohort) and 186 with Wavefront Optimized® LASIK (Control Cohort). No adverse events occurred during the postoperative period of the wavefront-guided LASIK procedures. In the Control Cohort, one subject undergoing traditional LASIK had the axis of astigmatism programmed as 115 degrees instead of the actual 155 degree axis. This led to cylinder in the left eye. The following complications were reported 6 months after wavefront-guided LASIK in the Study Cohort: 1.2% (2/166) of the eyes had a corneal epithelial defect; 1.2% (2/166) had foreign body sensation; and 0.6% (1/166) had pain. No complications were reported in the Control Cohort. Topography-Guided Myopia: There were six adverse events reported in the topography-guided myopia study. Four of the eyes experienced transient or temporary decreases in vision prior to the final 12 month follow-up visit, all of which were resolved by the final follow-up visit. One subject suffered from decreased vision in the treated eye, following blunt force trauma 4 days after surgery. One subject experienced retinal detachment, which was concluded to be unrelated to the surgical procedure. Clinical Data Myopia: The myopia clinical study included 901 eyes treated, of which 813 of 866 eligible eyes were followed for 12 months. Accountability at 3 months was 93.8%, at 6 months was 91.9%, and at 12 months was 93.9%. Of the 782 eyes that were eligible for the uncorrected visual acuity (UCVA) analysis of effectiveness at the 6-month stability time point, 98.3% were corrected to 20/40 or better, and 87.7% were corrected to 20/20 or better. Subjects who responded to a patient satisfaction questionnaire before and after LASIK reported the following visual symptoms at a “moderate” or “severe” level at least 1% higher at 3 months post-treatment than at baseline: visual fluctuations (28.6% vs. 12.8% at baseline). Long term risks of LASIK for myopia with and without astigmatism have not been studied beyond 12 months. Hyperopia: The hyperopia clinical study included 290 eyes treated, of which 100 of 290 eligible eyes were followed for 12 months. Accountability at 3 months was 95.2%, at 6 months was 93.9%, and at 12 months was 69.9%. Of the 212 eyes that were eligible for the UCVA analysis of effectiveness at the 6-month stability time point, 95.3% were corrected to 20/40 or better, and 69.4% were corrected to 20/20 or better. Subjects who responded to a patient satisfaction questionnaire before and after LASIK reported the following visual symptoms as “much worse” at 6 months post-treatment: halos (6.4%); visual fluctuations (6.1%); light sensitivity (4.9%); night driving glare (4.2%); and glare from bright lights (3.0%). Long term risks of LASIK for hyperopia with and without astigmatism have not been studied beyond 12 months. Mixed Astigmatism: The mixed astigmatism clinical study included 162 eyes treated, of which 111 were eligible to be followed for 6 months. Accountability at 1 month was 99.4%, at 3 months was 96.0%, and at 6 months was 100.0%. Of the 142 eyes that were eligible for the UCVA analysis of effectiveness at the 6-month stability time point, 97.3% achieved acuity of 20/40 or better, and 69.4% achieved acuity of 20/20 or better. Subjects who responded to a patient satisfaction questionnaire before and after LASIK reported the following visual symptoms at a “moderate” or “severe” level at least 1% higher at 3 months post-treatment than at baseline: sensitivity to light (52.9% vs. 43.3% at baseline); visual fluctuations (43.0% vs. 32.1% at baseline); and halos (42.3% vs. 37.0% at baseline). Long term risks of LASIK for mixed astigmatism have not been studied beyond 6 months. Wavefront-Guided Myopia: The wavefront-guided myopia clinical study included 374 eyes treated; 188 with wavefront-guided LASIK (Study Cohort) and 186 with Wavefront Optimized® LASIK (Control Cohort). 166 of the Study Cohort and 166 of the Control Cohort were eligible to be followed at 6 months. In the Study Cohort, accountability at 1 month was 96.8%, at 3 months was 96.8%, and at 6 months was 93.3%. In the Control Cohort, accountability at 1 month was 94.6%, at 3 months was 94.6%, and at 6 months was 92.2%. Of the 166 eyes in the Study Cohort that were eligible for the UCVA analysis of effectiveness at the 6-month stability time point, 99.4% were corrected to 20/40 or better, and 93.4% were corrected to 20/20 or better. Of the 166 eyes in the Control Cohort eligible for the UCVA analysis of effectiveness at the 6-month stability time point, 99.4% were corrected to 20/40 or better, and 92.8% were corrected to 20/20. In the Study Cohort, subjects who responded to a patient satisfaction questionnaire before and after LASIK reported the following visual symptoms at a “moderate” or “severe” level at least 1% higher at 3 months post-treatment than at baseline: light sensitivity (47.8% vs. 37.2% at baseline) and visual fluctuations (20.0% vs. 13.8% at baseline). In the Control Cohort, the following visual symptoms were reported at a “moderate” or “severe” level at least 1% higher at 3 months post-treatment than at baseline: halos (45.4% vs. 36.6% at baseline) and visual fluctuations (21.9% vs. 18.3% at baseline). Long term risks of wavefront-guided LASIK for myopia with and without astigmatism have not been studied beyond 6 months. Topography-Guided Myopia: The topography-guided myopia clinical study included 249 eyes treated, of which 230 eyes were followed for 12 months. Accountability at 3 months was 99.2%, at 6 months was 98.0%, and at 12 months was 92.4%. Of the 247 eyes that were eligible for the UCVA analysis at the 3-month stability time point, 99.2% were corrected to 20/40 or better, and 92.7% were corrected to 20/20 or better. Subjects who responded to a patient satisfaction questionnaire before and after LASIK reported the following visual symptoms as “marked” or “severe” at an incidence greater than 5% at 1 month after surgery: dryness (7% vs. 4% at baseline) and light sensitivity (7% vs. 5% at baseline). Visual symptoms continued to improve with time, and none of the visual symptoms were rated as being “marked” or “severe” with an incidence of at least 5% at 3 months or later after surgery. Long term risks of topography-guided LASIK for myopia with and without astigmatism have not been studied beyond 12 months. Information for Patients: Prior to undergoing LASIK surgery with a WaveLight® Excimer Laser System, prospective patients must receive a copy of the relevant Patient Information Booklet, and must be informed of the alternatives for correcting their vision, including (but not limited to) eyeglasses, contact lenses, photorefractive keratectomy, and other refractive surgeries. Attention: Please refer to a current WaveLight® Excimer Laser System Procedure Manual for a complete listing of the indications, complications, warnings, precautions, and side effects.
*Trademarks are the property of their respective owners.
1. Stulting R, Fant B. Results of topography-guided laser in situ keratomileusis custom ablation treatment with a refractive excimer laser. J Cataract Refract Surg. 2016;42(1):11-18. Study Description: Prospective, nonrandomized, multicenter study of 249 eyes with myopia or myopic astigmatism of 6.0 D or less. Outcome measures included manifest refraction, UDVA, CDVA and visual symptoms up to 12 months.
©2021 Alcon Inc. 9/21 US-TLV-2100003


