AIR OPTIX® plus HydraGlyde® Contact Lenses
Give Your Patients Outstanding Comfort from Day 1 to Day 301-3

Combine Two Unique Technologies into One Outstanding Lens

Comfort. Vision. Value.
Patients rated AIR OPTIX® plus HydraGlyde® a 9 out of 10 on initial insertion comfort.4,5

Two Unique Technologies
SmartShield® Technology Helps Deliver Superior Overall
Cholesterol Deposit Resistance Throughout the Lens4,6-8
SmartShield® Technology Creates an Ultra-thin Protective Layer to:
- Minimize the exposed silicone on the lens surface10
- Protect lenses from irritating deposits all month long6,7
- Help support a stable tear film and maintain wettability4,13
- AIR OPTIX® plus HydraGlyde® contact lenses with SmartShield® Technology helps resist lens parameter changes from everyday use of cosmetics, hand creams and makeup removers12,13,14
SmartShield® Technology Helps Deliver Superior Overall Cholesterol Deposit Resistance Throughout the Lens.4,6-8
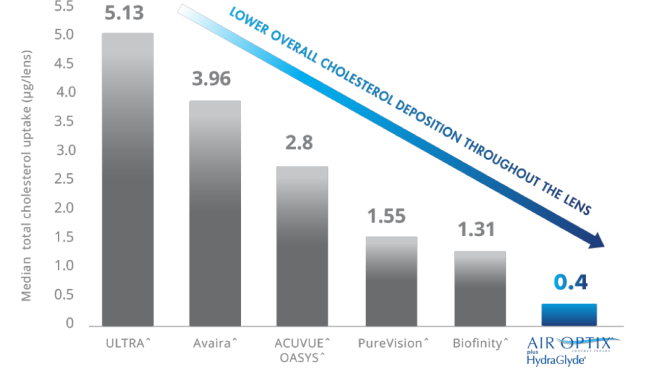
*Overall superior cholesterol deposit resistance of Air Optix® plus Hydraglyde® contact lenses, as compared to ACUVUE^ OASYS^ Lenses, PureVision^ lenses, Biofinity^ lenses and Avaira^ lenses, lenses worn daily for the manufacturer-recommended replacement period. CLEAR CARE® PLUS Cleaning & Disinfecting Solution used for cleaning and disinfection. all differences between AIR OPTIX® plus HydraGlyde® contact lenses and competitive brands statistically significant (p<0.05).
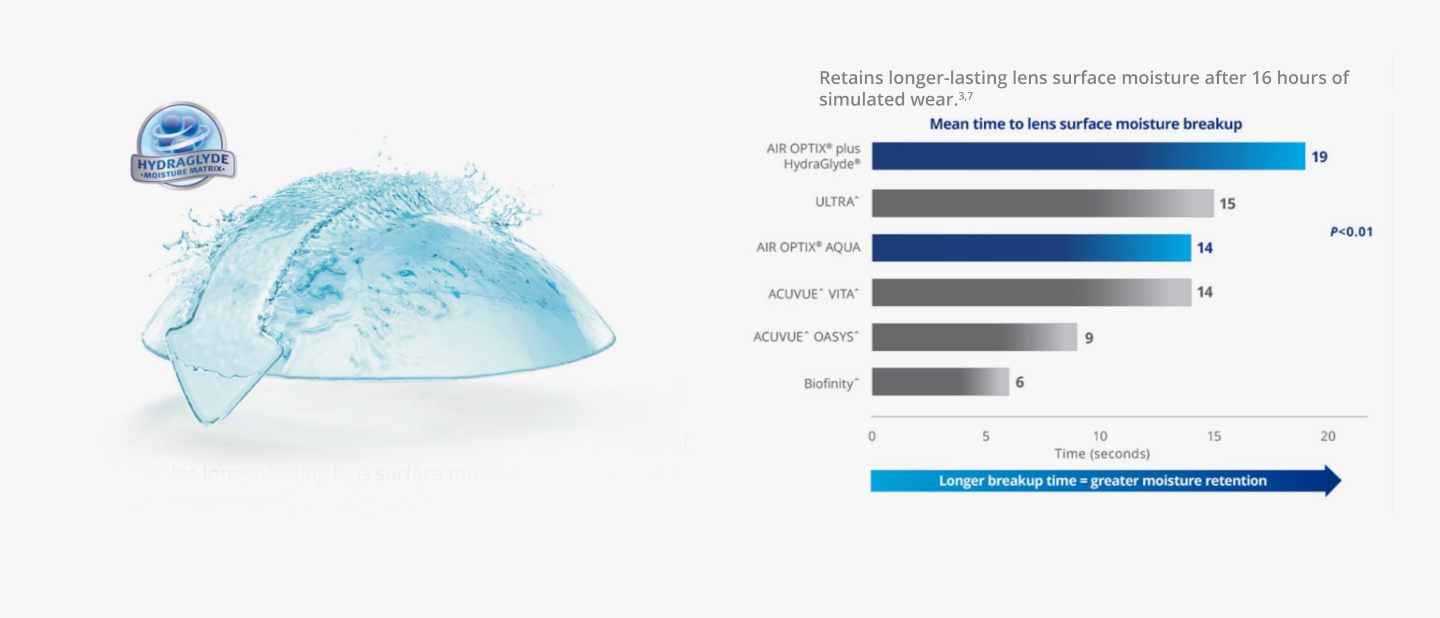
HydraGlyde® Moisture Matrix Technology surrounds lens
in long-lasting surface moisture to help keep the lens
surface hydrated
To Help Your Patients Get the Most From Their Contact Lenses, Recommend CLEAR CARE® PLUS and OPTI-FREE® Puremoist®

AIR OPTIX® plus HydraGlyde® Contact Lenses Product Information



AIR OPTIX® plus HydraGlyde® Multifocal
A multifocal contact lens designed to provide outstanding comfort from day 1 to day 30.1-3


AIR OPTIX® plus HydraGlyde® for Astigmatism
Outstanding stability with a predictable fit, and outstanding
comfort from day 1 to day 30 for your astigmatic patients.1-3,18
AIR OPTIX® plus Hydraglyde® Product Inserts and Guidelines
AIR OPTIX® plus HydraGlyde® Package Insert
AIR OPTIX® plus HydraGlyde® Fitting Guide
Reference Links
See product instructions for complete wear, care and safety information. ![]()
Important information for AIR OPTIX® plus HydraGlyde® Astigmatism (lotrafilcon B) contact lenses:
For daily wear or extended wear up to 6 nights for near/far-sightedness, presbyopia and/or astigmatism. Risk of serious eye problems (i.e., corneal ulcer) is greater for extended wear. In rare cases, loss of vision may result. Side effects like discomfort, mild burning or stinging may occur.
Important information for AIR OPTIX® plus HydraGlyde® (lotrafilcon B) contact lenses:
For daily wear or extended wear up to 6 nights for near/far-sightedness. Risk of serious eye problems (i.e., corneal ulcer) is greater for extended wear. In rare cases, loss of vision may result. Side effects like discomfort, mild burning or stinging may occur.
Important information for AIR OPTIX® plus HydraGlyde® Multifocal (lotrafilcon B) contact lenses:
For daily wear or extended wear up to 6 nights for near/far-sightedness. Risk of serious eye problems (i.e., corneal ulcer) is greater for extended wear. In rare cases, loss of vision may result. Side effects like discomfort, mild burning or stinging may occur.
^Trademarks are the property of their respective owners.
- Eiden SB, Davis RL, Bergenske PD. Prospective study of lotrafilcon B lenses comparing 2 versus 4 weeks of wear for objective and subjective measures of health, comfort, and vision. Eye & Contact Lens. 2013;39(4):290-294.
- Based on a 30-day clinical study of 75 habitual lotrafilcon B lens wearers; Alcon data on file, 2017.
- Lemp J, Kern J. A comparison of real time and recall comfort assessments. Optom Vis Sci. 2016;93:Eabstract 165256.
- Lemp J, Kern J. On-eye performance of lotrafilcon B lenses packaged with a substantive wetting agent. Poster presented at Optometry’s Meeting, the Annual Meeting of the American Optometric Association; June 21-25, 2017; Washington, D.C.
- In a randomized, double-masked clinical study with 337 patients at 25 sites; CIBA VISION data on file, 2008.
- Nash W, Gabriel M, Mowrey-Mckee M. A comparison of various silicone hydrogel lenses; lipid and protein deposition as a result of daily wear. Optom Vis Sci. 2010;87: E-abstract 105110.
- Nash W. Gabriel M. Ex vivo analysis of cholesterol deposition for commercially available silicone hydrogel contact lenses using a fluorometric enzymatic assay. Eye Contact Lens. 2014;40(5):277-282.
- Alcon data on file, 2016.
- Alcon data on file, 2013.
- Rex J, Perry S, Lemp J. Concentrations of silicon at silicone hydrogel contact lens surfaces. Poster presented and BCLA Clinical Conference; May 29-31, 2015; Liverpool, England.
- Guillon M, Maissa C, Wong S, Patel K, Lemp J. Tear film dynamics over silicone hydrogel contact lenses with different lipid deposition profiles. Optom Vis Sci. 2014;91:E-abstract 145196.
- Luensmann D, Yu M, Yang J, Srinivasan S, Jones L. Impact of Cosmetics on the Physical Dimension and Optical Performance of Silicone Hydrogel Contact Lenses. Eye & Contact Lens. 2015;4(4):218-227.
- Srinivasan S, Otchere H, Yu M, Yang J, Luensmann D, Jones L. Impact of Cosmetics on the surface properties of silicone hydrogen contact lenses. Eye & Contact Lens. 2015;41(4):228-235.
- Luensmann D, van Doorn K, May C, Srinivasan S, Jones L. Physical Dimension and Optical Assessment of Currently Marketed Silicone Hydrogel Contact Lenses After Exposure to Cosmetics. American Academy of Optometry, San Antonio, USA, 2018.
- In vitro study over 16 hours, to measure wetting substantivity; Alcon data on file, 2015.
- Muya L, Lemp J, Kern JR, Sentell KB, Lane J, Perry SS. Impact of packaging saline wetting agents on wetting substantivity and lubricity. Invest Opthalmol Vis Sci. 2016;57:E-abstract 1463.
- Tucker R, Lemp J, Guillon M. In vitro and on eye wettability of lotrafilcon B lenses packaged with a substantive wetting agent. Invest Ophthalmol Vis Sci. 2017;58(8):3070.
- In a masked, multi-site clinical study with over 150 patients; significance demonstrated at the 0.05 level; Alcon data on file, 2005.
