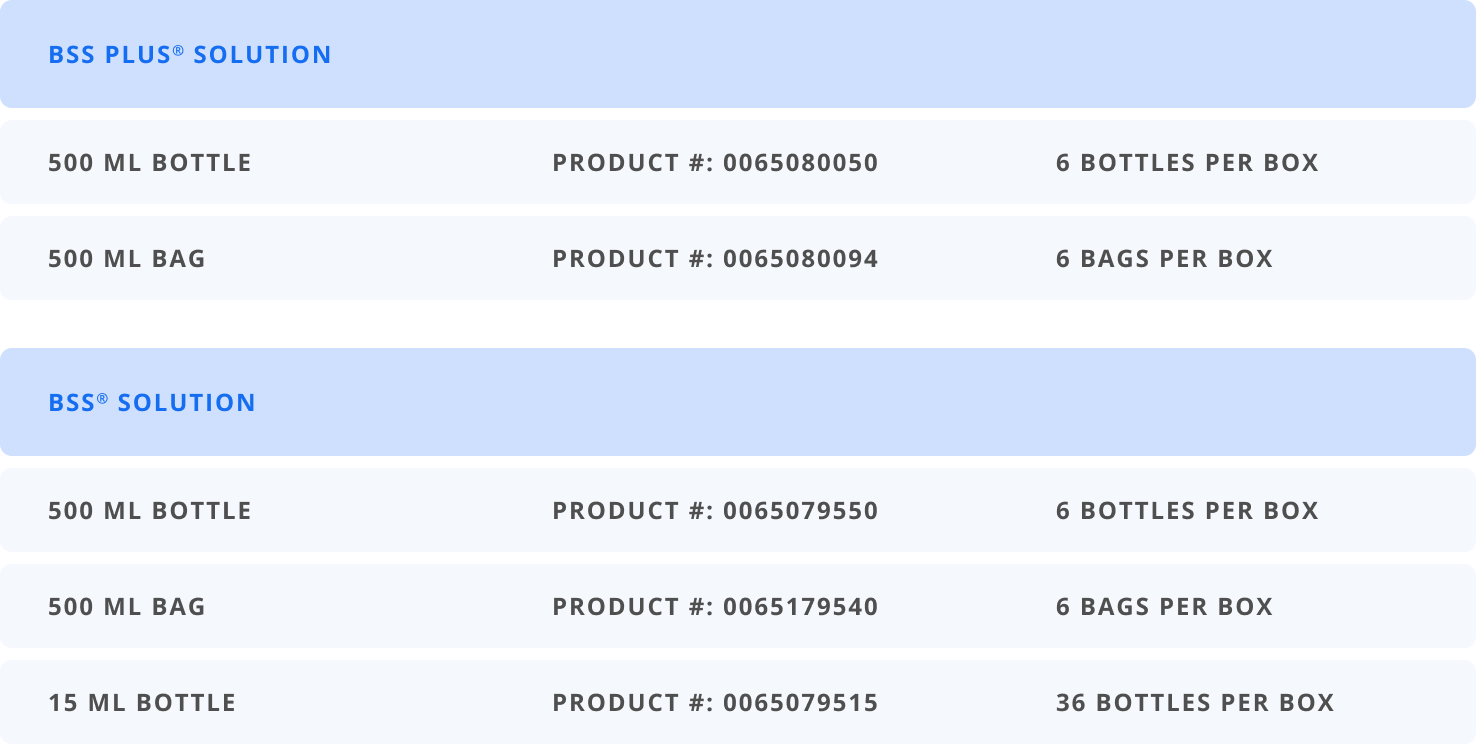
Intraocular Irrigating Solutions
BSS® PLUS Sterile Intraocular Irrigating Solution (balanced salt solution enriched with bicarbonate, dextrose, and glutathione).
BSS® Sterile Irrigating Solution (balanced salt solution).

BSS® Plus Solution


BSS® Solution
Explore More Solutions

Cataract Surgery

Vitreoretinal Procedures

Viscoelastics
Custom Pak® Advantage
Your procedure is our priority. With an Alcon Custom Pak®, you can bundle BSS PLUS® and BSS® Solutions with all of the other items you need, so you can go into every procedure feeling confident and ready.

IMPORTANT SAFETY INFORMATION
BSS® STERILE IRRIGATING SOLUTION (BALANCED SALT SOLUTION)
INDICATION AND DOSING
BSS® Sterile Irrigating Solution is indicated for use as an extraocular and intraocular irrigating solution during ocular surgical procedure involving perfusion of the eye with an expected maximum duration of less that 60 minutes.
DOSAGE AND ADMINISTRATION
This irrigating solution should be used according to standard format for each surgical procedure. Follow directions of the particular administration set to be used. Remove the outer overwrap. Clean and disinfect the rubber stopper by using a sterile alcohol wipe. Insert the spike aseptically into the bag through the target area of the rubber stopper. Allow the fluid to flow and remove air from the tubing before irrigation begins.
WARNINGS AND PRECAUTIONS
NOT FOR INJECTION OR INTRAVENOUS INFUSION. Do not use unless outer overwrap is intact, product is clear, seal is intact and bag is undamaged. Do not use if product is discolored or contains a precipitate. SINGLE patient use only. The contents of this bag should not be used in more than one patient. This solution contains no preservative, unused contents should be discarded.
Open under aseptic conditions only. Studies suggest that intraocular irrigating solutions which are iso-osmotic with normal aqueous fluids should be used with caution in diabetic patients undergoing vitrectomy since intraoperative lens changes have been observed. There have been reports of corneal clouding and edema following ocular surgery in which BSS Sterile Irrigating Solution was used as an irrigating solution.
ADVERSE REACTIONS
Irrigation or any other trauma may result in cornel swelling or bullous keratopathy. Post-operative inflammatory reactions as well as incidents of corneal edema and corneal decompensation have been reported.
For additional information on BSS® Sterile Irrigating Solution, please click here for the full Prescribing Information.
BSS PLUS® STERILE INTRAOCULAR IRRIGATING SOLUTION (BALANCED SALT SOLUTION ENRICHED WITH BICARBONATE, DEXTROSE, AND GLUTATHIONE)
INDICATION AND DOSING
BSS PLUS® Irrigating Solution is indicated for use as an intraocular irrigating solution during intraocular surgical procedures involving perfusion of the eye. Dosage and Administration does not contain a separate airway tube. Follow the directions for the particular administration set to be used. Insert the spike aseptically into the bottle through the center target area of the rubber stopper. Allow the fluid to flow to remove air from the tubing before intraocular irrigation begins. If a second bottle is necessary to complete the surgical procedure, ensure that the vacuum is vented from the second bottle BEFORE attachment to the administration set.
CONTRAINDICATIONS
There are no specific contraindications to the use of BSS PLUS®; however, contraindications for the surgical procedure during which BSS PLUS® is to be used should be strictly adhered to.
WARNINGS AND PRECAUTIONS
For IRRIGATION during ophthalmic surgery only. Not for injection of intravenous infusion. Do not use unless product is clear, seal is intact, vacuum is present and container is undamaged. Do not use if product is discolored or contains a precipitate.
DO NOT USE BSS PLUS® UNTIL PART I IS FULLY RECONSTITUED WITH PART II. Discard unused contents. BSS PLUS® does not contain a preservative; therefore, do not use this container for more than one patient. DISCARD ANY UNUSED PORTION SIX HOURS AFTER PREPARATION. Studies suggest that intraocular irrigating solutions which are iso-osmotic with normal aqueous fluids should be used with caution in diabetic patients undergoing vitrectomy since intraoperative lens changes have been observed.
There have been reports of corneal clouding or edema following ocular surgery in which BSS PLUS® was used as an irrigating solution. As in all surgical procedures, appropriate measures should be taken minimize trauma to the cornea and other ocular tissues.
ADVERSE REACTIONS
Postoperative inflammatory reactions as well as incidents of corneal edema and corneal decompensation have been reported. Their relationship to the use of BSS PLUS® has not been established.
For additional information on BSS® Sterile Irrigating Solution, please click here for the full Prescribing Information.