Specialists in Retina
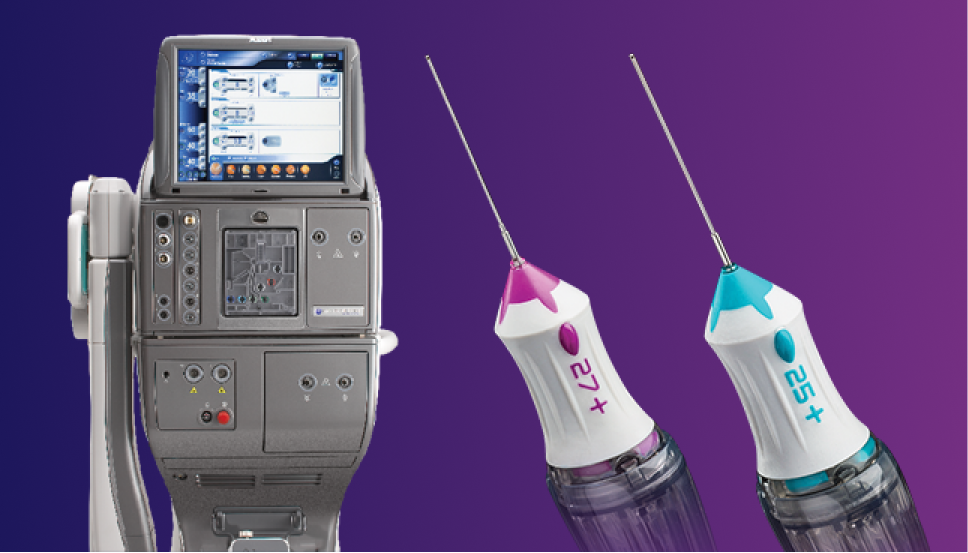
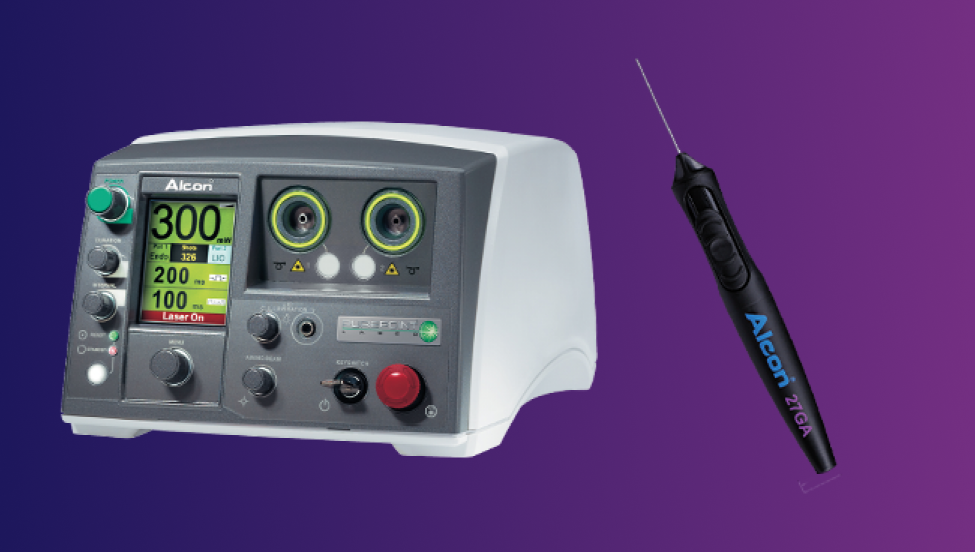
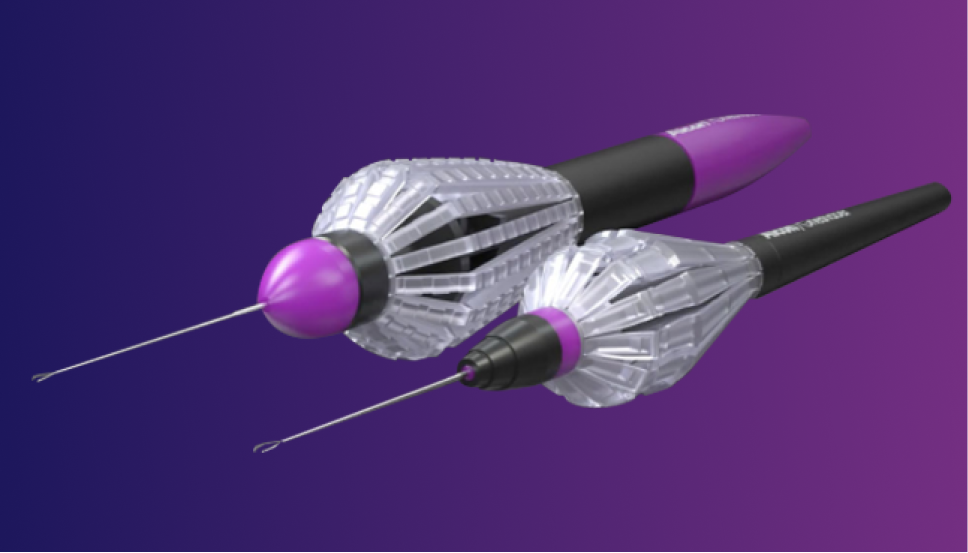
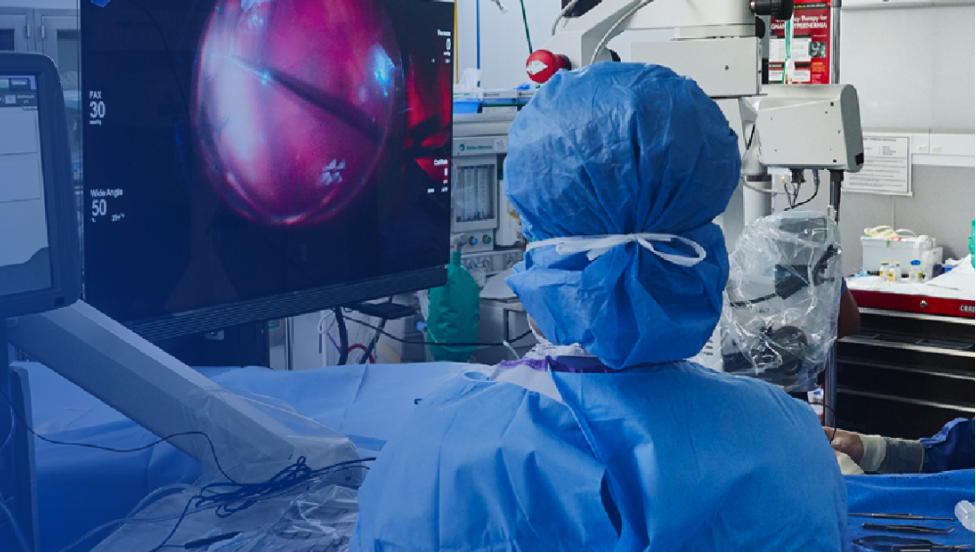
Precision and Integration for Retina Surgery
Alcon Experience Academy
For relevant training content from industry thought leaders
Please refer to relevant products DFU or Operator’s manuals for complete list of indications, contraindications and warnings
© 2023 Alcon Inc. 7/23 US-VIT-2300020