Ophthalmic Viscosurgical Devices (OVDs)
Protect what’s important.

Protect Effectively with Chondroitin Sulfate
OVDs with chondroitin sulfate lose viscosity at a slower rate during stress and readily adhere to ocular tissue:1
![]() Better Endothelial Cell Protection1,4,6,7
Better Endothelial Cell Protection1,4,6,7
![]() Superior Clarity and Visualization3,7
Superior Clarity and Visualization3,7
![]() Superior Retention During Lens Removal1
Superior Retention During Lens Removal1
![]() Superior Anterior Capsule Dome Maintenance3
Superior Anterior Capsule Dome Maintenance3
![]() Proven Mechanical Protection of Space Maintenance3
Proven Mechanical Protection of Space Maintenance3
![]() Easy to Remove after Procedure3
Easy to Remove after Procedure3

DuoVisc® System
Operate confidently in everyday cases and complex situations

DisCoVisc® OVD
Designed for use through an entire cataract surgery
One System. No Compromises.
DuoVisc® Viscoelastic System
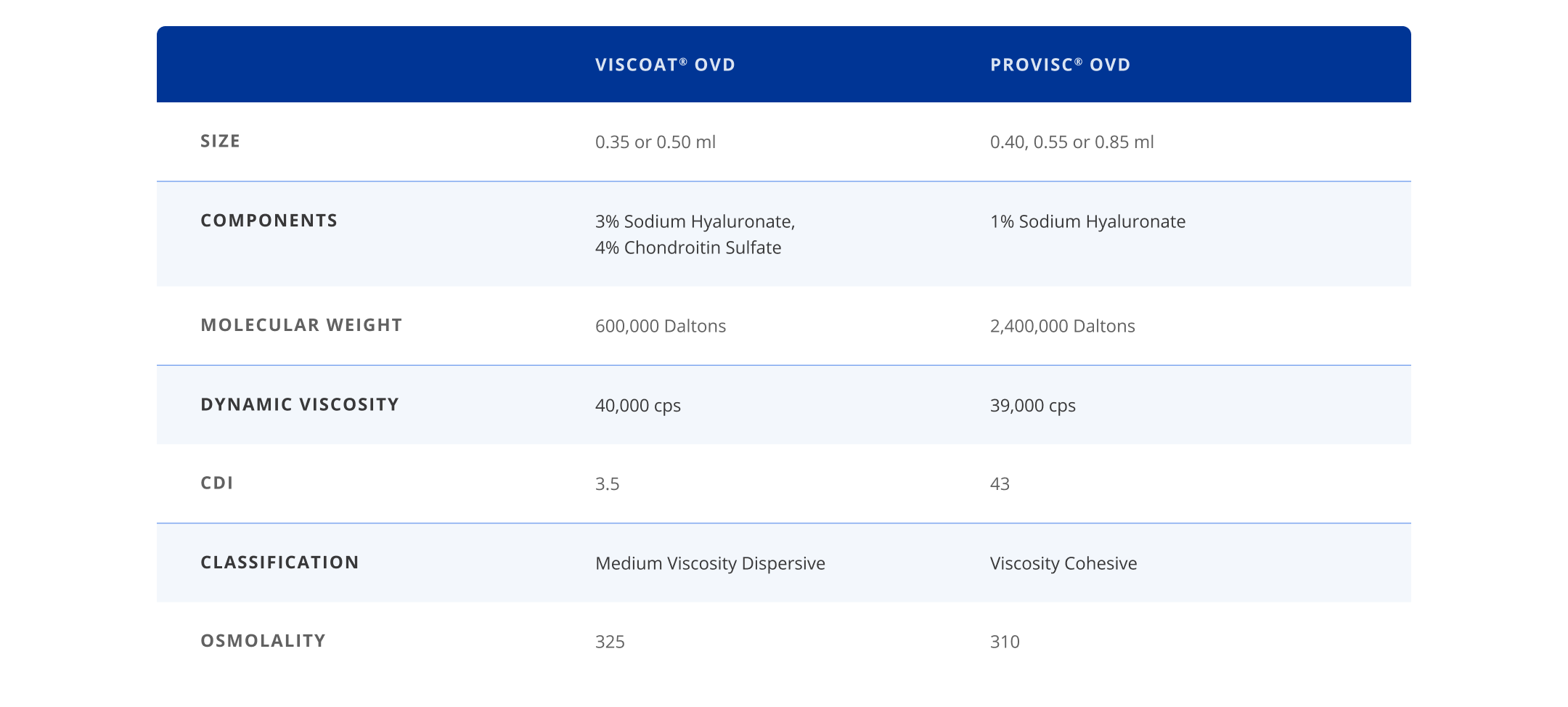
DuoVisc® Viscoelastic System offers both the endothelial protection of chondroitin sulfate in VISCOAT® OVD (dispersive) with the proven mechanical protection and space maintenance found in PROVISC® OVD (cohesive)..

VISCOAT® OVD for Proven Protection
The only dispersive OVD offering the protection of 4% Chondroitin Sulfate.
High molecular weight to maximize space maintenance in the anterior chamber and capsular bag.
Greater binding to corneal endothelium due to two extra negative charges from Chondroitin Sulfate, totaling three negative charges,4,5 versus one in most OVDs.


PROVISC® OVD for Mechanical Protection and Space Maintenance
High molecular weight to maximize space maintenance in the anterior chamber and capsular bag.3
Excellent clarity and easy removal.
One Viscoelastic. Full Procedure.
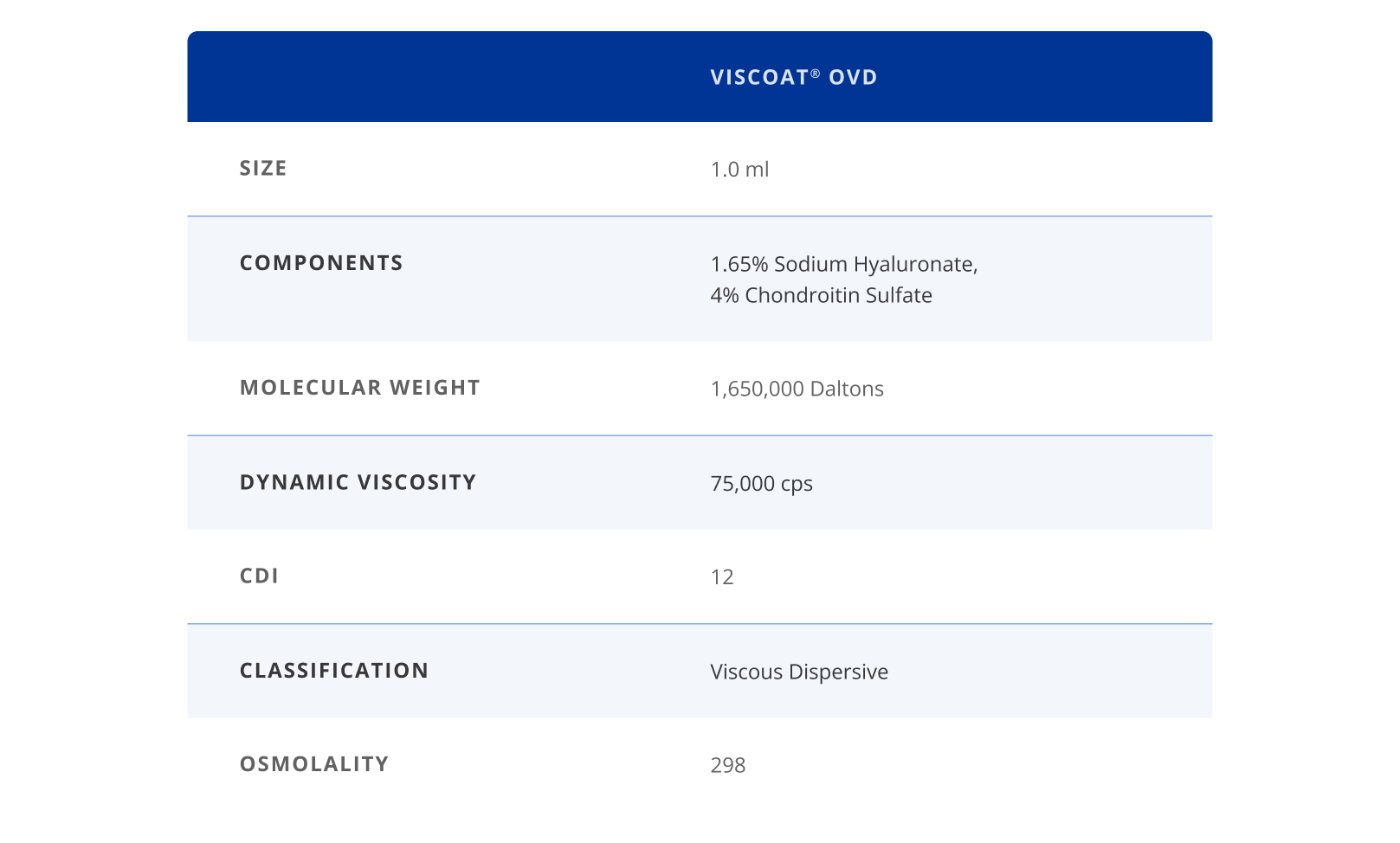
DisCoVisc® OVD: The First Viscous Dispersive
DisCoVisc®OVD, the first and only viscous dispersive, provides the flexibility of both cohesive and dispersive properties in a single syringe. Its chemical and mechanical design provides genuine endothelial protection, as well as better access and clarity.3

Excellence at Each Stage


at 6mL/min aspiration rate
full space maintenance during IOL insertion vs 0 μ when using competitors1
Retention during lens removal (phaco) phase4
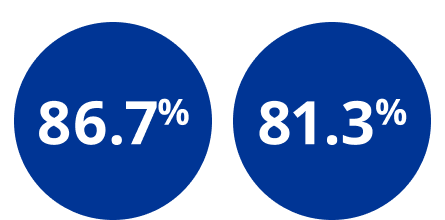

full space maintenance during IOL insertion vs 47.9% when using competitor*1 *Competitor is Healon | chamber maintenance during capsulotomy
|
Space maintenance during capsulorhexis, and IOL insertion phases3


excellent or good visualization during the surgical procedure
vs 88.1% when using competitor
Visualization during the surgical procedure3
Product Details
DuoVisc® Viscoelastic System
DisCoVisc® OVD
Important Product Information for DisCoVisc® OVD
Caution: Federal (USA) law restricts this device to sale by or on the order of a physician.
Indications: DisCoVisc® (Sodium Chondroitin Sulfate-Sodium Hyaluronate) Ophthalmic Viscosurgical Device (OVD) is indicated for use during surgery in the anterior segment of the eye. It is designed to create and maintain space, to protect the corneal endothelium and other intraocular tissues and to manipulate tissues during surgery. It may also be used to coat intraocular lenses and instruments during cataract extraction and IOL insertion.
Warnings/Precautions: Failure to follow assembly instructions or use of an alternate cannula may result in cannula detachment and potential patient injury. Precautions are limited to those normally associated with the surgical procedure being performed. Although sodium hyaluronate and sodium chondroitin sulfate are highly purified biological polymers, the physician should be aware of the potential allergic risks inherent in the use of any biological material.
Adverse Reactions: DisCoVisc® Ophthalmic Viscosurgical Device was very well tolerated in nonclinical and clinical studies. A transient rise in intraocular pressure in the early postoperative period may be expected due to the presence of sodium hyaluronate, which has been shown to affect such a rise. It is therefore recommended that DisCoVisc® OVD be removed from the anterior chamber by thorough irrigation and/or aspiration at the end of surgery to minimize postoperative IOP increases.
ATTENTION: Reference the Directions for Use for a complete listing of warnings and precautions.
Important Product Information for DUOVISC®, VISCOAT®, PROVISC® OVD
Description: DUOVISC® Viscoelastic System is designed to provide two Viscoelastic materials with different physico-chemical properties that can be used differently and/or sequentially to perform specific tasks during a cataract procedure. DUOVISC® Viscoelastic System consists of VISCOAT® Ophthalmic Viscosurgical Device and PROVISC® Ophthalmic Viscosurgical Device.
Caution: Federal (USA) law restricts this device to sale by, or on the order of, a physician.
Description: VISCOAT® (Sodium Chondroitin Sulfate – Sodium Hyaluronate) Ophthalmic Viscosurgical Device
Indications: VISCOAT® OVD is indicated for use as an ophthalmic surgical aid in anterior segment procedures including cataract extraction and intraocular lens (IOL) implantation. VISCOAT® OVD maintains a deep anterior chamber during anterior segment surgeries, enhances visualization during the surgical procedure, and protects the corneal endothelium and other ocular tissues. The viscoelasticity of the solution maintains the normal position of the vitreous face and prevents formation of a flat chamber during surgery.
Warnings/Precautions: Failure to follow assembly instructions or use of an alternate cannula may result in cannula detachment and potential patient injury. Precautions are limited to those normally associated with the surgical procedure being performed. Although sodium hyaluronate and sodium chondroitin sulfate are highly purified biological polymers, the physician should be aware of the potential allergic risks inherent in the use of any biological material.
Adverse Reactions: VISCOAT® OVD has been extremely well tolerated in human and animal studies. A transient rise in intraocular pressure in the early postoperative period may be expected due to the presence of sodium hyaluronate, which has been shown to affect such a rise. It is therefore recommended that VISCOAT® OVD be removed from the anterior chamber by thorough irrigation and/or aspiration at the end of surgery to minimize postoperative IOP increases. Do not overfill anterior chamber.
ATTENTION: Please refer to the Directions for Use for a complete listing of indications, warnings and precautions.
Description: PROVISC® (Sodium Hyaluronate) Ophthalmic Viscosurgical Device
Indications: PROVISC® OVD is indicated for use as an ophthalmic surgical aid in the anterior segment during cataract extraction and intraocular lens (IOL) implantation. Ophthalmic viscoelastics serve to maintain a deep anterior chamber during anterior segment surgery allowing reduced trauma to the corneal endothelium and surrounding ocular tissues. They help push back the vitreous face and prevent formation of a flat chamber during surgery.
Warnings/Precautions: Postoperative increases in intraocular pressure have been reported with sodium hyaluronate products. The IOP should be carefully monitored and appropriate therapy instituted if
significant increases should occur. It is recommended that PROVISC® OVD be removed by irrigation and/or aspiration at the close of surgery. Do not overfill anterior chamber. Although sodium hyaluronate is a highly purified biological polymer, the physician should be aware of the potential allergic risks inherent in the use of any biological material; care should be used in patients with hypersensitivity to any components in this material. Cannula assembly instructions should be followed to prevent patient injury.
Adverse Reactions: Postoperative inflammatory reactions such as hypopyon and iritis have been reported with the use of ophthalmic viscoelastics, as well as incidents of corneal edema, corneal decompensation, and a transient rise in intraocular pressure.
ATTENTION: Please refer to the directions for use for a complete listing of indications, warnings and precautions
References
1. Petroll WM, et al. Quantitative assessment of ophthalmicviscosurgical device retention using invivo confocal microscopy. J Cataract Refract Surg. 2005 Dec;31(12):2363-8.
2. Vasavada A, et al. Protective effect of ophthalmic viscosurgical devices (OVDs) against hydrogen peroxide-induced oxidative damage to corneal endothelial cells: an in-vitro model. Accepted for presentation: American Society of Cataract and Refractive Surgeons; April 3-8, 2--9; SAn Francisco, CA.
3. DisCoVisc® OVD Product Insert
4. Glasser B, et al. Protective Effects of Viscous Solutions in Phacoemulsifaction
5. Poyer J, et al. New Method to measure the retention of viscoelastic agents on a rabbit corneal
6. Lindstrom, et al. Protective Effect of OVDs Against Hydrogen Peroxide-Induced Oxidative Damage
7. Papconstantinou, et al. Clinical trial evaluation Viscoat and Visthesia ophthalmic viscosurgical
