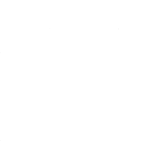
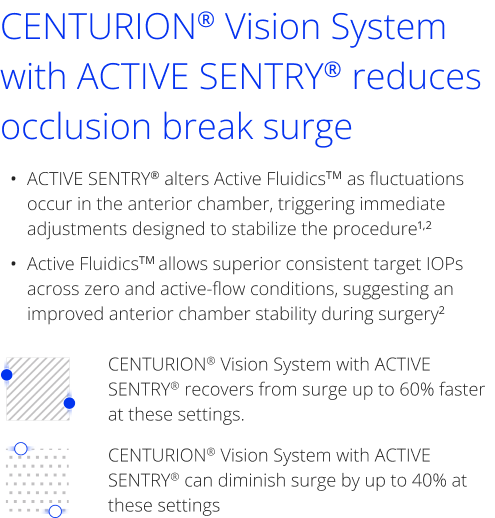
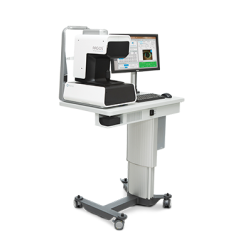
CENTURION® Vision System with ACTIVE SENTRY®
Safer in every moment1-4



CENTURION® Vision System with ACTIVE SENTRY® offers safety, stability, and consistency to cataract surgeons1-4
CENTURION® Vision System with ACTIVE SENTRY® is designed with advanced technology to help enhance confidence during surgery with lower, more physiological IOP and enhanced chamber stability, even in challenging cases.1-4
- The ACTIVE SENTRY® handpiece is the first and only phacoemulsification handpiece with a built-in pressure sensor5,6
- ACTIVE SENTRY® technology helps reduce post-occlusion surge and maintain stability in the anterior chamber by adjusting for IOP fluctuations7,8

CENTURION® Vision System with ACTIVE SENTRY® allows surgeons to operate at a lower, more physiological IOP and significantly higher vacuum levels5-8
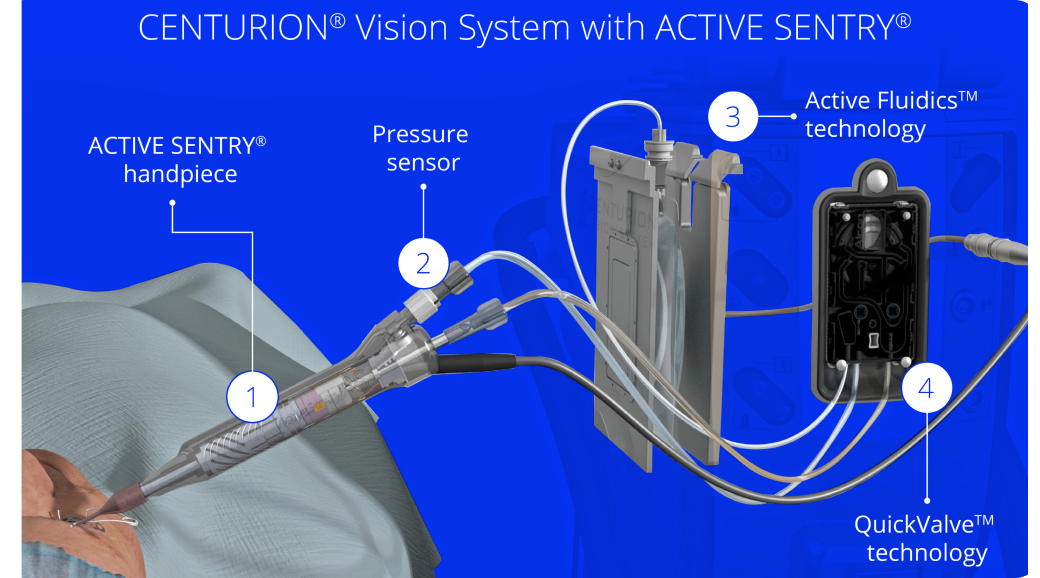

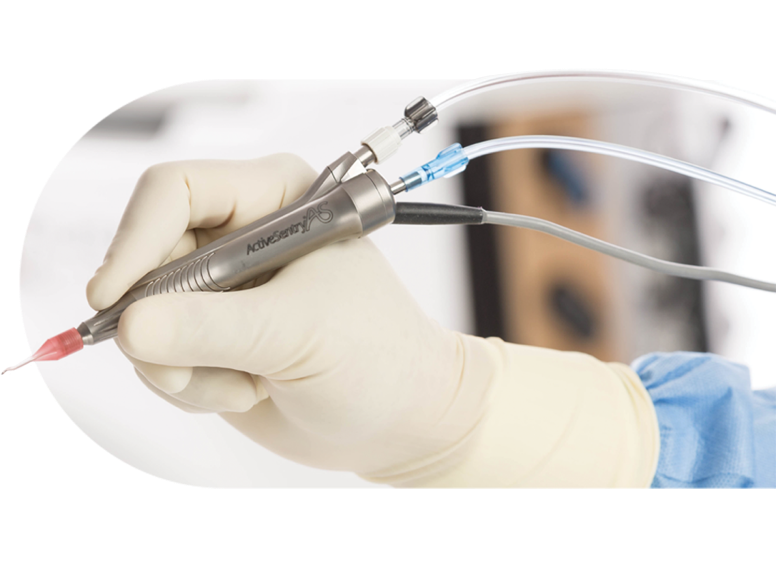
ACTIVE SENTRY® handpiece
Signals to CENTURION® hardware and software that adjustments are needed to maintain consistent IOP.

Pressure sensor
Detects changes in anterior chamber stability as they occur.

Active FluidicsTM technology
Uses compression plates to adjust pressure on BSS® irrigating solution bag, compensating for changes in the eye.

QuickValveTM technology
Releases fluid into aspiration line and minimizes occlusion break.
ACTIVE SENTRY® presents unprecedented responsiveness
- Facilitates operating at near physiological IOP, which supports ocular safety9
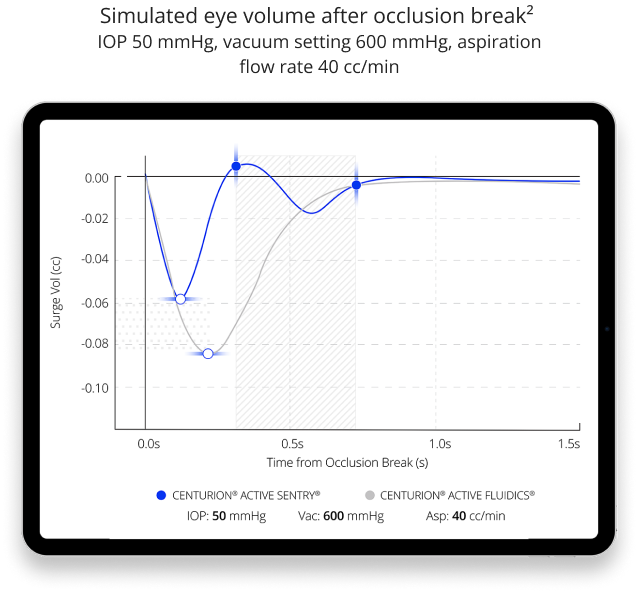
- Reduces post-occlusion surge7
- Controls for real-time IOP fluctuations8
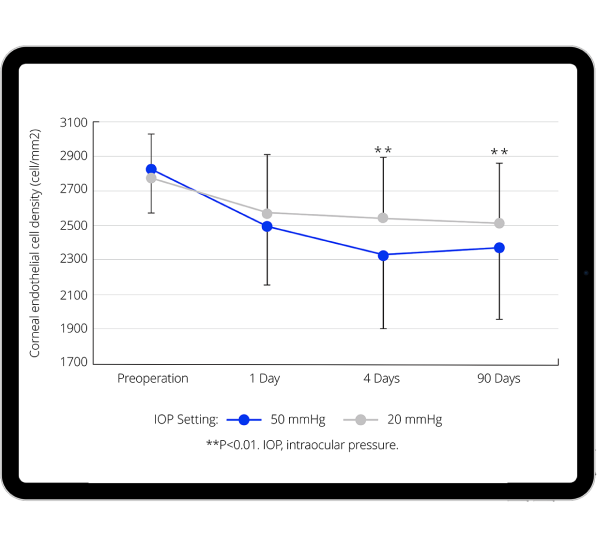
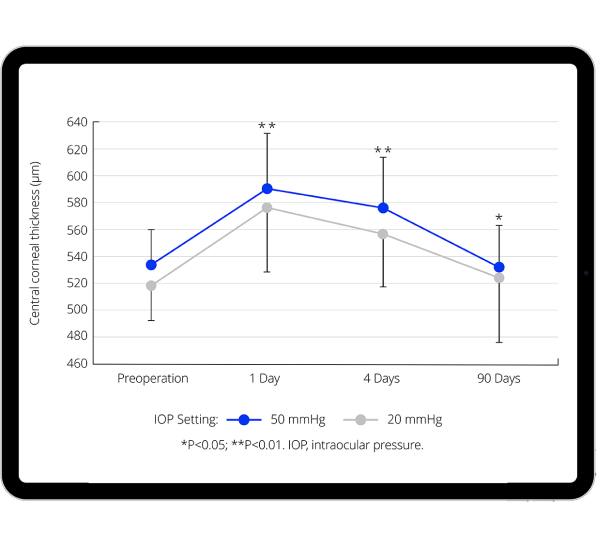
Benefits of lower, more physiological IOP on anterior segment
Recent evidence shows that more physiological IOP during cataract surgery is associated with:
- Decrease in corneal edema.10-12
- Reduced increase in central corneal thickness.9,11,12
- Decrease in the presence of Descemet's folds.11,12
- Decrease in anterior segment inflammation (cells & flare) on day 1 post-op.11,12
- Increase in corneal clarity in the early post-op period.11,12
- Evidence indicates that near physiological IOP results in less endothelial cell loss.10,13-17
- Supporting comfort for the patient.19,20

CENTURION® Vision System with ACTIVE SENTRY® provides the most stable chamber surgeons have ever experienced
CENTURION® ENERGY DELIVERY
- Elegant performance. Accelerated cataract removal21*
- Enhanced torsional efficiency21,22
- CENTURION® Energy Delivery is designed to set the global standard for efficiency in the OR
*As compared with the INFINITI® Vision System.

Optimized to help procedure efficiency
CENTURION® Vision System with ACTIVE SENTRY® significantly reduced CDE and U/S time by 21.8% and 28.7%, respectively23
Efficacy outcomes23
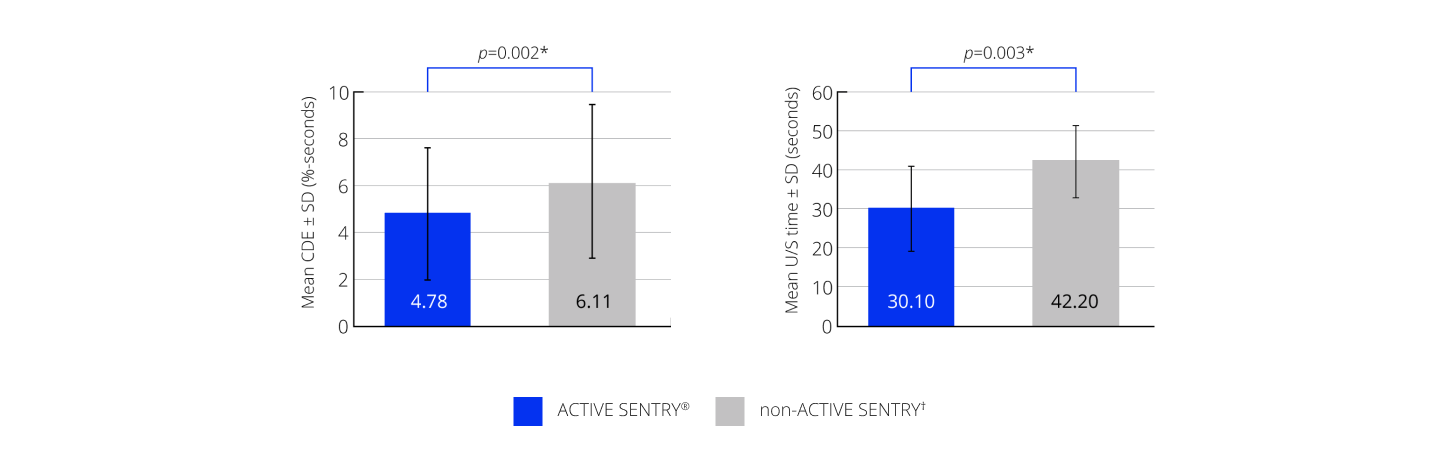
*A p-value of 0.005 or less was considered statistically significant; †non-ACTIVE SENTRY is also referred to as OZil® handpiece. CDE, cumulative dissipated energy; SD, standard deviation; U/S, ultrasound.
Reduced heat transfer to the eye during surgery
The BALANCED Tip produced less heat than the mini-flared and bent-mini tips, demonstrating the smallest temperature rise of <11° Celsius at all test conditions.24
Temperature rise under free-flow conditions and occlusion using a 30-g load† and increasing amplitudes24
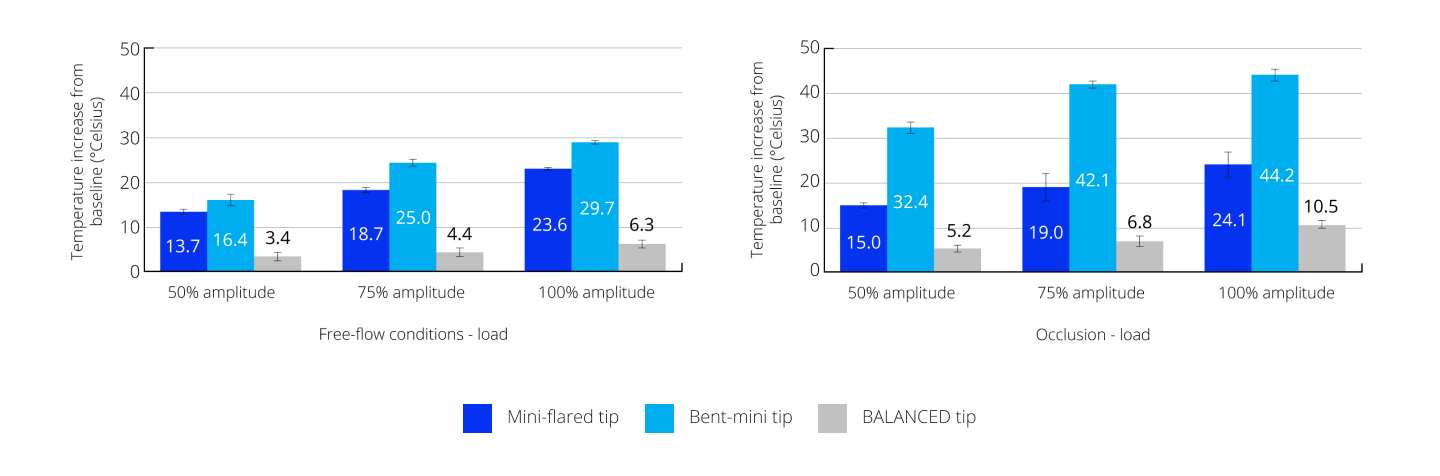
†The 30-g load was approximately applied where an estimated or probable incision location would be to simulate friction at the incision site. IOP, intraocular pressure.

More consistency across phaco procedures
CENTURION® Vision System with ACTIVE SENTRY® integrates with the Alcon Cataract Refractive Suite for leading-edge surgical procedures, enhanced by safety, stability, and efficiency.1-4


ARGOS® Image Guided System
Surgical Planning


LuxOR® RevaliaTM Ophthalmic Microscope
Anterior and posterior segment visualization

NGENUITY® 3D Visualization System
Anterior and posterior segment visualization

LenSX® Laser
Laser incisions and fragmentation


ORA SYSTEM® with VerifEyeTM Lynk
Intraoperative
Aberrometry

CENTURION® Vision System with ACTIVE SENTRY®
Phacoemulsification

Most implanted IOL platform1
Cataract surgery
Experience the benefits of CENTURION® Vision System with ACTIVE SENTRY®

ACTIVE SENTRY® maintains stability in the anterior chamber by adjusting for IOP fluctuations8
ACTIVE SENTRY® facilitates operating at lower, more physiological IOP, potentially supporting a comfortable procedure for patients6,9
ACTIVE SENTRY® reduces occlusion break surge, mitigates incision leakage, and automatically adjusts PEL7,25,26
The efficiency of CENTURION® with BALANCED tip* was significantly increased across all lens opacification grades, as compared with longitudinal U/S mode27
The Hybrid tip reduces PCR thanks to its proprietary polymer technology28

ACTIVE SENTRY® has a short learning curve in experienced phacoemulsification surgeons6
Related products


Custom-Pak® Surgical Pack
Your procedure is our priority. Custom-Pak® Surgical Pack keeps you ready by bundling your customized toolkit—complete with phaco handpieces, tips, sleeves, and more.

INTREPID® Hybrid Tip
Designed to reduce damage to the capsular bag and other tissues29

OVDs
Alcon OVDs contain chondroitin sulfate, allowing slower viscosity loss during stress and better endothelial cell protection.30


INTREPID® Transformer I/A
Specifically designed for easy transition from either coaxial or bimanual cortical removal without changing handpieces.

ARGOS® Image
Guided System


LuxOR® RevaliaTM
Ophthalmic Microscope

NGENUITY® 3D
Visualization System

LenSX® Laser


ORA SYSTEM®
with VerifEyeTM Lynk
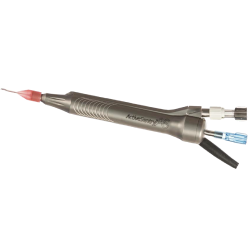

ACTIVE SENTRY®
Handpiece
Alcon Experience Academy
A non-promotional training and education resource for eye care professionals.
CENTURION® VISION SYSTEM IMPORTANT PRODUCT INFORMATION
Caution: Federal (USA) law restricts this device to sale by, or on the order of, a physician.
As part of a properly maintained surgical environment, it is recommended that a backup IOL Injector be made available in the event the AutoSert® IOL Injector Handpiece does not perform as expected.
Indication: The Centurion® Vision System is indicated for emulsification, separation, irrigation, and aspiration of cataracts, residual cortical material and lens epithelial cells, vitreous aspiration and cutting associated with anterior vitrectomy, bipolar coagulation, and intraocular lens injection. The AutoSert® IOL Injector Handpiece is intended to deliver qualified AcrySof® intraocular lenses into the eye following cataract removal.
The AutoSert® IOL Injector Handpiece achieves the functionality of injection of intraocular lenses. The AutoSert® IOL Injector Handpiece is indicated for use with the AcrySof® lenses SN6OWF, SN6AD1, SN6AT3 through SN6AT9, as well as approved AcrySof® lenses that are specifically indicated for use with this inserter, as indicated in the approved labeling of those lenses.
Warnings: Appropriate use of Centurion® Vision System parameters and accessories is important for successful procedures. Use of low vacuum limits, low flow rates, low bottle heights, high power settings, extended power usage, power usage during occlusion conditions (beeping tones), failure to sufficiently aspirate viscoelastic prior to using power, excessively tight incisions, and combinations of the above actions may result in significant temperature increases at incision site and inside the eye, and lead to severe thermal eye tissue damage.
Good clinical practice dictates the testing for adequate irrigation and aspiration flow prior to entering the eye. Ensure that tubings are not occluded or pinched during any phase of operation.
The consumables used in conjunction with ALCON® instrument products constitute a complete surgical system. Use of consumables and handpieces other than those manufactured by Alcon may affect system performance and create potential hazards.
AEs/Complications: Inadvertent actuation of Prime or Tune while a handpiece is in the eye can create a hazardous condition that may result in patient injury. During any ultrasonic procedure, metal particles may result from inadvertent touching of the ultrasonic tip with a second instrument. Another potential source of metal particles resulting from any ultrasonic handpiece may be the result of ultrasonic energy causing micro abrasion of the ultrasonic tip.
ATTENTION: Refer to the Directions for Use and Operator’s Manual for a complete listing of indications, warnings, cautions and notes.
References
- CENTURION® Vision System User Manual.
- Alcon Data on File, 2017.
- Thorne A, Dyk DW, Fanney D, Miller KM. Phacoemulsifier occlusion break surge volume reduction. J Cataract Refract Surg. 2018;44(12):1491-1496.
- Aravena C, Dyk DW, Thorne A, Fanney D, Miller KM. Aqueous volume loss associated with occlusion break surge in phacoemulsifiers from 4 different manufacturers. J Cataract Refract Surg. 2018;44(7):884-888.
- Alcon Vision LLC. 2019. Alcon launches advancements to the CENTURION® Vision System at American Society for Cataract and Refractive Surgery Meeting. Available at: https://www.alcon.com/media-release/alcon-launches-advancements-centurionr-vision-system-american-society-cataract-and. Accessed Feb 2022.
- Cyril D, et al. Active Sentry versus OZil hand piece: A prospective randomized comparative study. J Cataract Refract Surg. 2021;48:328.
- Miller KM, et al. Experimental study of occlusion break surge volume in 3 different phacoemulsification systems. J Cataract Refract Surg. 2021:47;1466.
- Vasavada V et al. Real-time dynamic changes in intraocular pressure after occlusion break: Comparing 2 phacoemulsification systems. J Cataract Refract Surg. 2021;47:1205.
- Kokubun T et al. Verification for the usefulness of normal tension cataract surgery. JOS 2022. Oral presentation.
- Suzuki H, et al. Effect of bottle height on the corneal endothelium during phacoemulsification. J Cataract Refract Surg. 2009;35:2014-2017.
- Vasavada AR, et al. Impact of high and low aspiration parameters on postoperative outcomes of phacoemulsification: Randomized clinical trial. J Cataract Refract Surg. 2010;36:588-593.
- Vasavada V, et al. Real-time dynamic intraocular pressure fluctuations during microcoaxial phacoemulsification using different aspiration flow rates and their impact on early postoperative outcomes: A randomized clinical trial. J Refract Surg. 2014;30(8):534-540.
- Kokubun T, et al. The protective effect of normal-IOP cataract surgery on the corneal endothelium. 25th Annual Meeting of the Japanese Ophthalmological Society.
- Takhtaev YV, Kiseleva TN & Shliakman RB. The effect of preset intraoperative intraocular pressure during phacoemulsification on the blood flow velocity in the central retinal artery. Ophthalmology Journal. 2019;12:5-12.
- Chen D, et al. Effect of simulated dynamic intraocular pressure on retinal thickness measured by optical coherence tomography after cataract surgery. Int J Ophthalmol. 2012;5(6):687-693.
- Li T, et al. Influence of cataract surgery on macular vascular density in patients with myopia using optical coherence tomography angiography. Exp Ther Med. 2020;20:258.
- Liu X, et al. Dynamic changes in retinal vessel density observed by optical coherence tomography angiography after phacoemulsification: active vs gravity fluidics system. Arq Bras Oftalmol. 2022;85:205-207.
- Vasavada V, et al. Impact of fluidic parameters during phacoemulsification on the anterior vitreous face behaviour: Experimental study. Indian J Ophthalmol. 2019:67(10):1634-1637.
- Hou, CH et al. The sources of pain during phacoemulsification using topical anesthesia. Eye (Lond). 2012;26:749-750.
- O’Brien PD, Fulcher T, Wallace D & Power W. Patient pain during different stages of phacoemulsification using topical anesthesia. J Cataract Refract Surg. 2001;27:880-883.
- Solomon K, Lorente R, Cionni R, Fanney D. Prospective, randomized clinical study using a new phaco system with intraocular system target pressure control. ASCRS-ASOA Symposium and Congress; April 25-29, 2014; Boston, USA.
- Zacharias J. Comparative motion profile characterization of the mini flared and balanced phacoemulsification tips. ESCRS; September 5-9, 2015; Barcelona, Spain.
- Jirásková N & Stepanov A. Our experience with Active Sentry and Centurion Ozil handpieces. Czech and Slovak Ophthalmology. 2021;77(1):18-21.
- Zacharías J. Laboratory assessment of thermal characteristics of three phacoemulsifcation tip designs operated using torsional ultrasound. Clin Ophthalmol. 2016;10:1095-1101.
- Crandall AS. Role of incision leakage in anterior chamber stability in different phacoemulsifier systems. ASCRS 2019. Oral presentation.
- Lehmann R. Automated patient eye level by sensor-based handpiece. ASCRS 2019. Oral presentation.
- Dasgupta S & Mehra R. Comparative studies between longitudinal and torsional modes in phacoemulsification, using active fluidics technology along with the intrepid balanced tip. Indian J Ophthalmol 2018;66:1417-1422.
- Shumway K et al. Evaluation of the capsular safety of a new hybrid phacoemulsification tip in a cadaver eye model. J Cataract Refract Surg 2019;45:1660-1664.
- Alcon Data on File.
