Clareon® Collection IOLs
More options for more patients: four optic designs, one glistening-free* material1,2
Clareon® Collection IOLs
More options for more patients: four optic designs, one glistening-free* material1,2
The Clareon® IOL portfolio offers the latest material advancement in a 20+ year history of continual innovation in IOLs.
Presbyopia-Mitigating IOLs
Help reduce the need for glasses at all distances3-5 on a glistening-free* BioMaterial.1,2

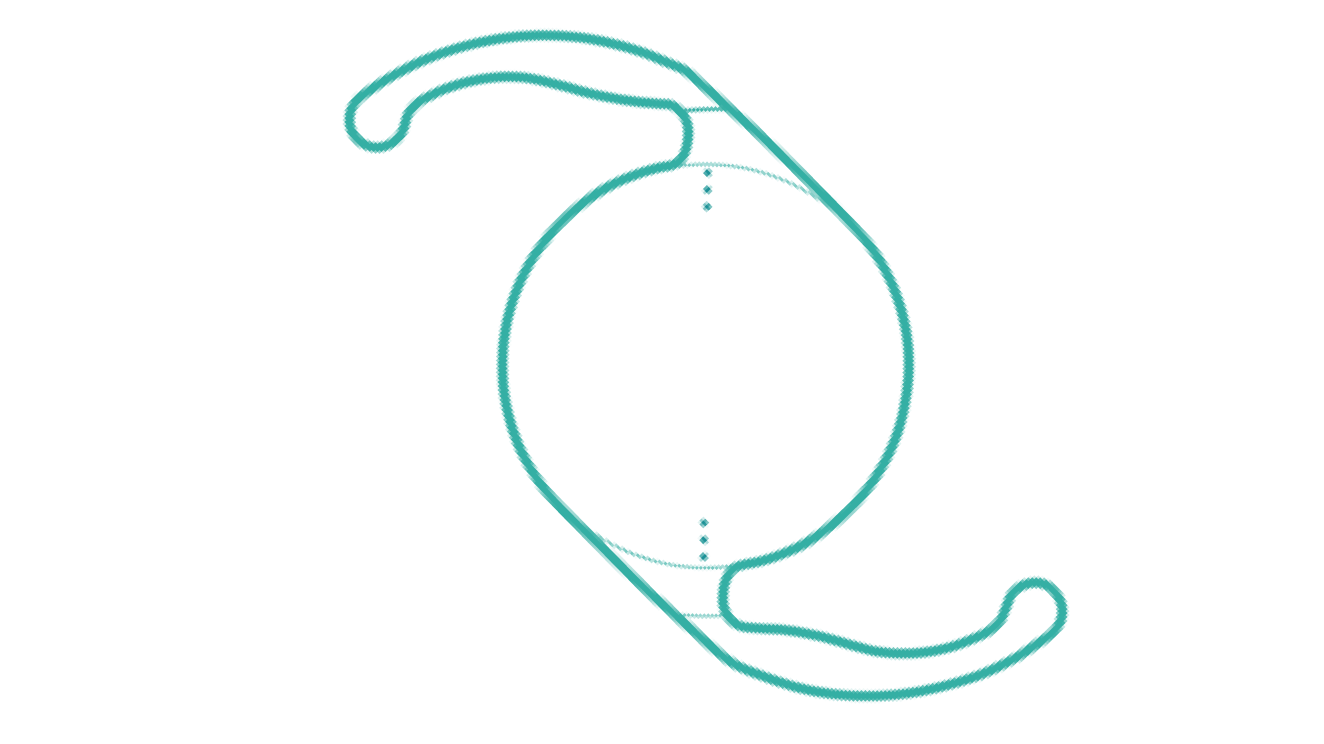
Clareon® PanOptix® IOL
The first and only trifocal lens in the United States, offering a full range of vision and exceptional clarity.2,3**

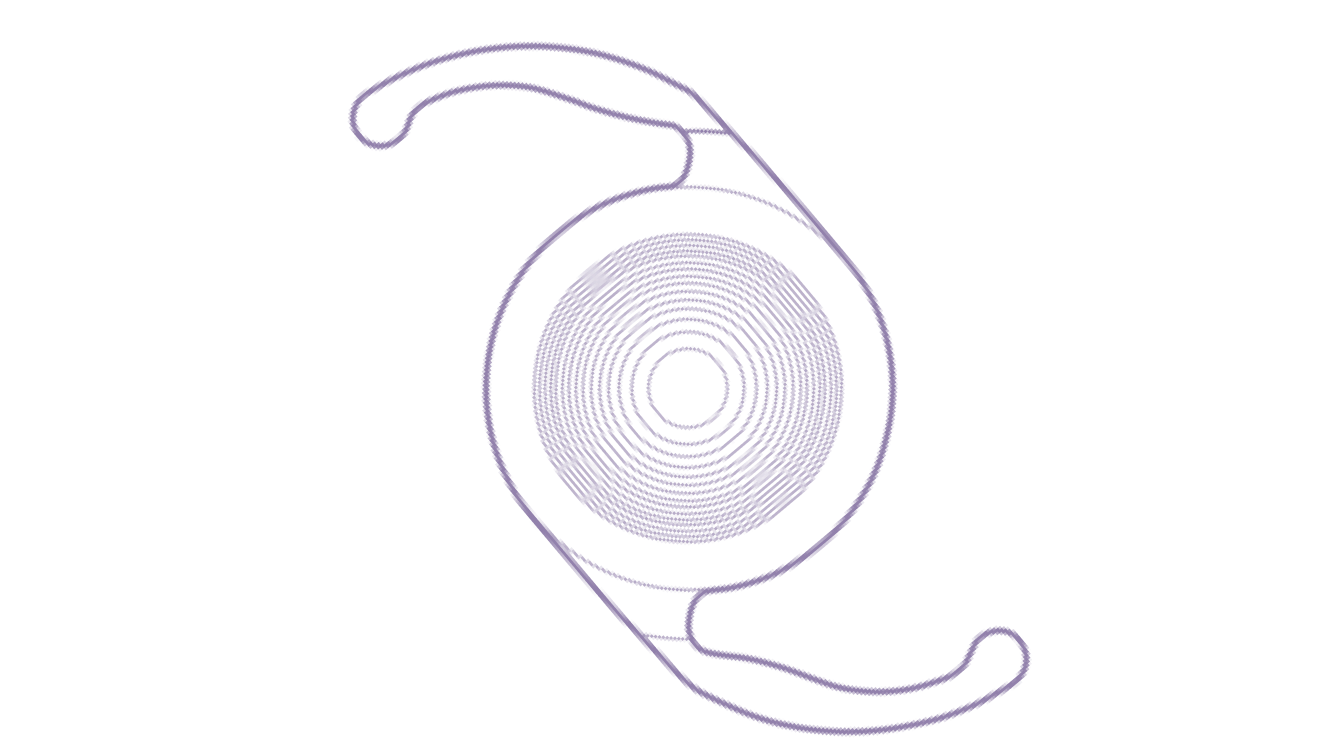
Clareon® Vivity® IOL
The first and only non-diffractive presbyopia-mitigating technology with exceptional clarity.1,5*
Monofocal IOLs
Glistening-free* BioMaterial with outstanding clarity**, for sharp, crisp vision.1,2,6

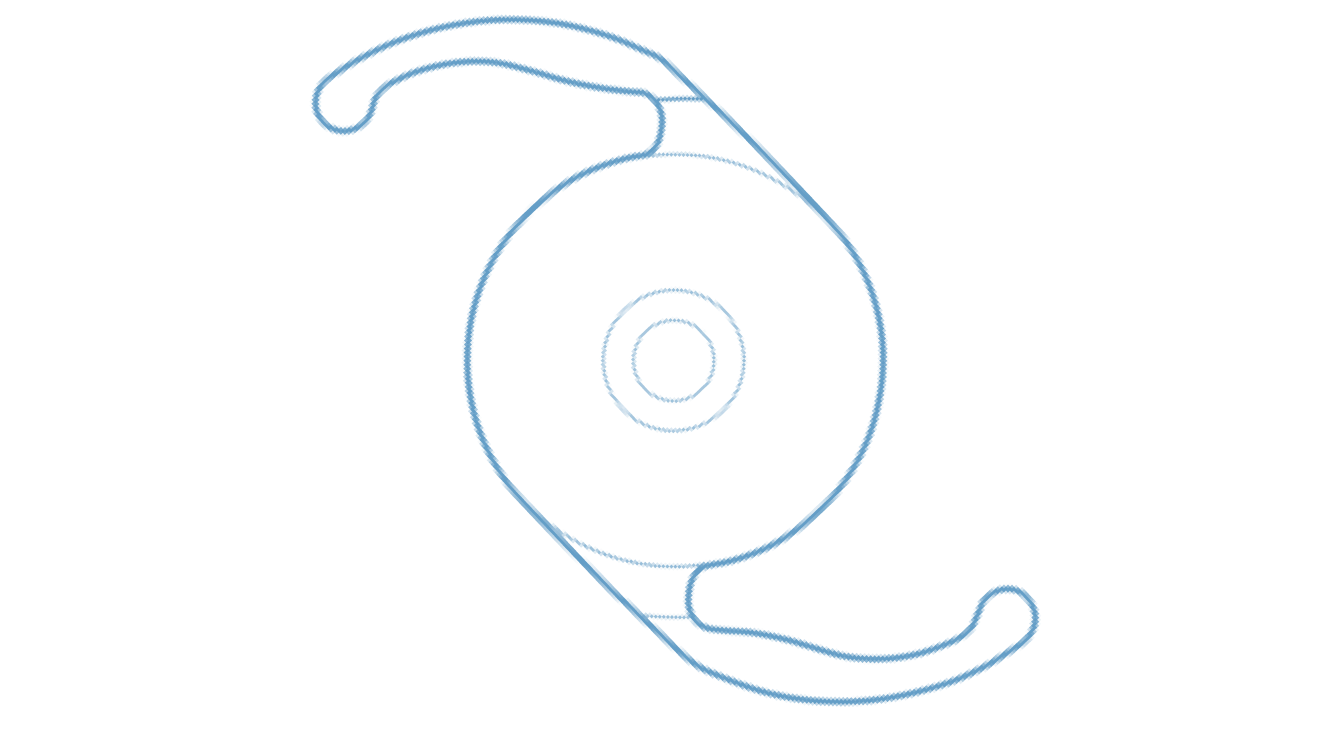
Clareon® Monofocal IOL
Exceptional clarity,** allowing you to confidently deliver long-lasting refractive outcomes.1,2
The latest evolution of control and clarity with the Clareon® Monarch® IV and Clareon® AutonoMe® IOL delivery systems
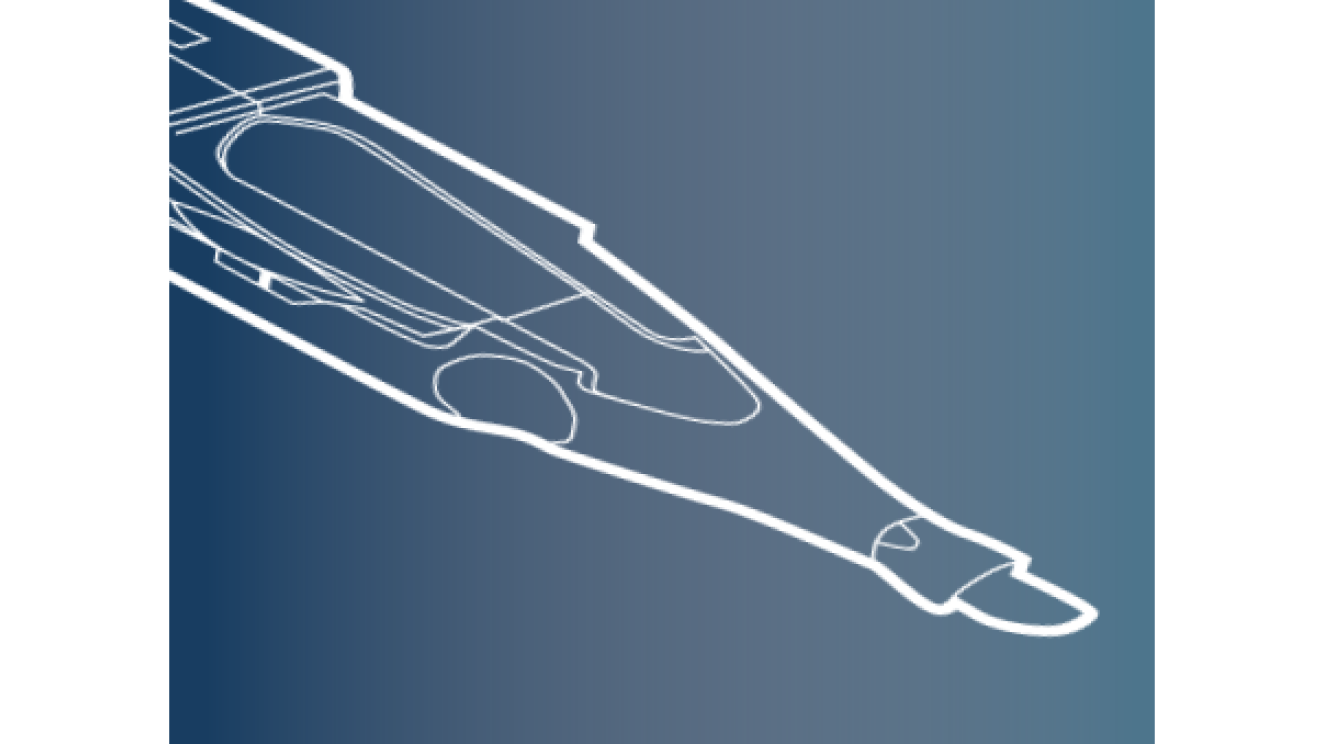
Clareon® Monarch® IV Delivery System
Monarch® IV manual delivery system designed specifically for Clareon® IOLs provides reliability, control and user comfort.8,9
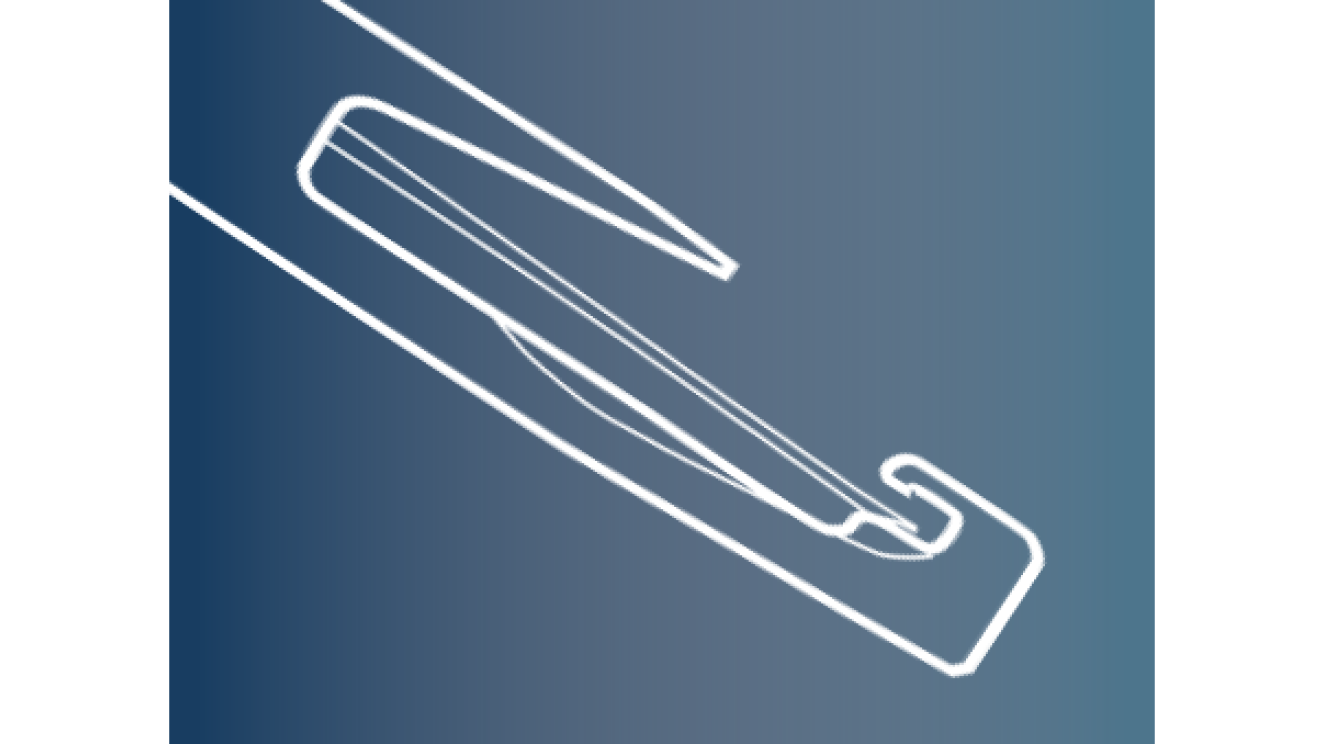
Clareon® AutonoMe® IOL Delivery System
AutonoMe® preloaded IOL delivery system provides easy, intuitive control for precise IOL insertion.10
See the difference Clareon® can make
An advanced manufacturing process combines a proprietary precision edge design with the exceptional BioOptics and BioMechanics of all Alcon IOLs.11
Peer perspectives on Clareon®
Learn more about your peers' experience with the Clareon® Collection of IOLs.

AVAILABLE ON CLAREON®: ALCON'S MOST ADVANCED IOL BIOMATERIAL TO DATE



*Defined as modified Miyata grade 0, <25mv /mm2 over 3 years (n=138), and over 9 years (n=20), respectively.
**Based on in vitro examinations of glistenings, surface haze and SSNGs.
IMPORTANT PRODUCT INFORMATION - CLAREON® FAMILY OF IOLS
CAUTION: Restricted by law to sale by or on the order of a physician.
INDICATIONS: The family of Clareon® intraocular lenses (IOLs) includes the Clareon® Aspheric Hydrophobic Acrylic and Clareon® Aspheric Toric IOLs, the Clareon® PanOptix® Trifocal Hydrophobic IOL, Clareon® PanOptix® Toric, Clareon® Vivity™ Extended Vision Hydrophobic Posterior Chamber IOL and Clareon® Vivity™ Toric IOLs. Each of these IOLs is indicated for visual correction of aphakia in adult patients following cataract surgery. In addition, the Clareon® Toric IOLs are indicated to correct pre-existing corneal astigmatism at the time of cataract surgery. The Clareon® PanOptix® lens mitigates the effects of presbyopia by providing improved intermediate and near visual acuity, while maintaining comparable distance visual acuity with a reduced need for eyeglasses, compared to a monofocal IOL. The Clareon® Vivity™ lens mitigates the effects of presbyopia by providing an extended depth of focus. Compared to an aspheric monofocal IOL, the lens provides improved intermediate and near visual acuity, while maintaining comparable distance visual acuity. All of these IOLs are intended for placement in the capsular bag.
WARNINGS / PRECAUTIONS: General cautions for all Clareon® IOLs: Careful preoperative evaluation and sound clinical judgment should be used by the surgeon to decide the risk/benefit ratio before implanting any IOL in a patient with any of the conditions described in the Directions for Use that accompany each IOL. Physicians should target emmetropia, and ensure that IOL centration is achieved. For the Clareon® Aspheric Toric, PanOptix® Toric and Vivity™ Toric IOLs, the lens should not be implanted if the posterior capsule is ruptured, if the zonules are damaged, or if a primary posterior capsulotomy is planned. Rotation can reduce astigmatic correction; if necessary lens repositioning should occur as early as possible prior to lens encapsulation. For the Clareon® PanOptix® IOL, some visual effects may be expected due to the superposition of focused and unfocused multiple images. These may include some perceptions of halos or starbursts, as well as other visual symptoms. As with other multifocal IOLs, there is a possibility that visual symptoms may be significant enough that the patient will request explant of the multifocal IOL. A reduction in contrast sensitivity as compared to a monofocal IOL may be experienced by some patients and may be more prevalent in low lighting conditions. Therefore, patients implanted with multifocal IOLs should exercise caution when driving at night or in poor visibility conditions. Patients should be advised that unexpected outcomes could lead to continued spectacle dependence or the need for secondary surgical intervention (e.g., intraocular lens replacement or repositioning). As with other multifocal IOLs, patients may need glasses when reading small print or looking at small objects. Posterior capsule opacification (PCO), may significantly affect the vision of patients with multifocal IOLs sooner in its progression than patients with monofocal IOLs. For the Clareon® Vivity™ IOL, most patients implanted with the Vivity™ IOL are likely to experience significant loss of contrast sensitivity as compared to a monofocal IOL. Therefore, it is essential that prospective patients be fully informed of this risk before giving their consent for implantation of the Clareon® Vivity™ IOL. In addition, patients should be warned that they will need to exercise caution when engaging in activities that require good vision in dimly lit environments, such as driving at night or in poor visibility conditions, especially in the presence of oncoming traffic. It is possible to experience very bothersome visual disturbances, significant enough that the patient could request explant of the IOL. In the parent AcrySof® IQ Vivity™ IOL clinical study, 1% to 2% of AcrySof® IQ Vivity™ IOL patients reported very bothersome starbursts, halos, blurred vision, or dark area visual disturbances; however, no explants were reported.Prior to surgery, physicians should provide prospective patients with a copy of the Patient Information Brochure available from Alcon informing them of possible risks and benefits associated with these IOLs.
ATTENTION: Reference the Directions for Use labeling for each IOL for a complete listing of indications, warnings and precautions.
REFERENCES:
- Werner L, Thatthamla I, Ong M, et al. Evaluation of clarity characteristics in a new hydrophobic acrylic IOL. J Cataract Refract Surg. 2019;45: 1490-1497.
- Lehmann R, Maxwell A, Lubeck DM, Fong R, Walters TR, Fakadej A. Effectiveness and Safety of the Clareon® Monofocal Intraocular Lens: Outcomes from a 2-Month Single-Arm Clinical Study in a Large Sample. Clin Ophthalmol. 2021;15:1647-1657. Published 2021 Apr 20.
- Clareon® PanOptix® Trifocal Hydrophobic Acrylic IOL Model CNWTT0 2021 Directions for Use.
- Zhu D, Ren S, Mills K, Hull J, Dhariwal M. Rate of Complete Spectacle Independence with a Trifocal Intraocular Lens: A Systematic Literature Review and Meta-Analysis. Ophthalmol Ther. 2023;12(2):1157-1171.
- Clareon® Vivity® Extended Vision Hydrophobic IOL (CNWET0) Directions for Use – USA.
- Clareon® Toric Directions for Use.
- Lee B, Chang D. Comparison of the rotational stability of two toric intraocular lenses in 1273 consecutive eyes. Ophthalmology. 2018;125(9):1325-1331.
- Alcon Data on File, 2019.
- Alcon Data on File, 2021.
- Clareon® AutonoMe® IOL Directions for Use.
- Das KK, Werner L, Collins S, Hong X. In vitro and schematic model eye assessment of glare or positive dysphotopsia-type photic phenomena:Comparison of a new material IOL to other monofocal IOLs. J Cataract Refract Surg. 2019;45(2):219-227.