Air Optix® Night & Day® Aqua
A flexible lens-wearing experience designed for up to 30 nights of continuous wear1,2*
*Extended wear for up to 30 continuous nights, as prescribed by an eye care professional.

Busy Patients Need Clear and Comfortable Vision, Whether It’s in the Middle of the Night…
…or first thing in the morning


Busy Professionals
Need flexibility to see clearly at anytime


Moms With Young Kids
Can’t see clearly getting up throughout the night


Unpredictable Work Schedules
Need immediate vision waking up for a shift


Frequent Travelers
Travel too often to hassle with my lenses
AIR OPTIX® NIGHT & DAY® AQUA Can Help These Patients With SmartShield® Technology and Ultimate Breathability3*
AIR OPTIX® NIGHT & DAY® AQUA contact lenses are approved by Health Canada for up to 30 days and nights of continuous wear.


SmartShield® Technology
Delivers a protective layer of moisture to help shield lenses from irritating deposits4,5


Highest Breathability3*
Oxygen continuously flows through the lens for white, healthy looking eyes3,6


A Winning Combination
More of your patients can wake up to comfortable, clear vision
*Dk/t = 175 @ -3.00D. Other factors may impact eye health.
Unique SmartShield® Technology Helps Offer Lasting Comfort, And Deposit Resistance4,5,7,8
AIR OPTIX® Night and Day® contact lenses are designed with SmartShield® Technology, to protect the lens, retain moisture, and resist deposits. This means lenses stay comfortable, day after day.4,5,9
Look Closer at a Contact Lens Surface Like no Other


SmartShield® Technology


Creates a Hydrophilic Environment


That Resists Lipids and Deposits
Superior Lipid Deposit Resistance4,5*†

*Lenses worn daily through manufacturer-recommended replacement period.
†CLEAR CARE® Cleaning & Disinfecting Solution used for cleaning and disinfection. Median values represented.
Superior Wettability10*
In vitro measurement of unworn lenses, median values represented, n=20.

*All differences between AIR OPTIX® NIGHT & DAY® AQUA contact lenses and competitive brands statistically significant (p<0.0001).
Compared to Biofinity^, PureVision^, ACUVUE^ OASYS, ACUVUE^ ADVANCE and ACAIRA^ contact lenses.
The Most Breathable Lens Material on the Market11-13

AIR OPTIX® NIGHT & DAY® AQUA has a Low Rate of Microbial Keratitis (MK)14
A rigorous post-market surveillance study of nearly 5,000 lotrafilcon A contact lens wearers for one year, and across 100+ clinical practices showed:
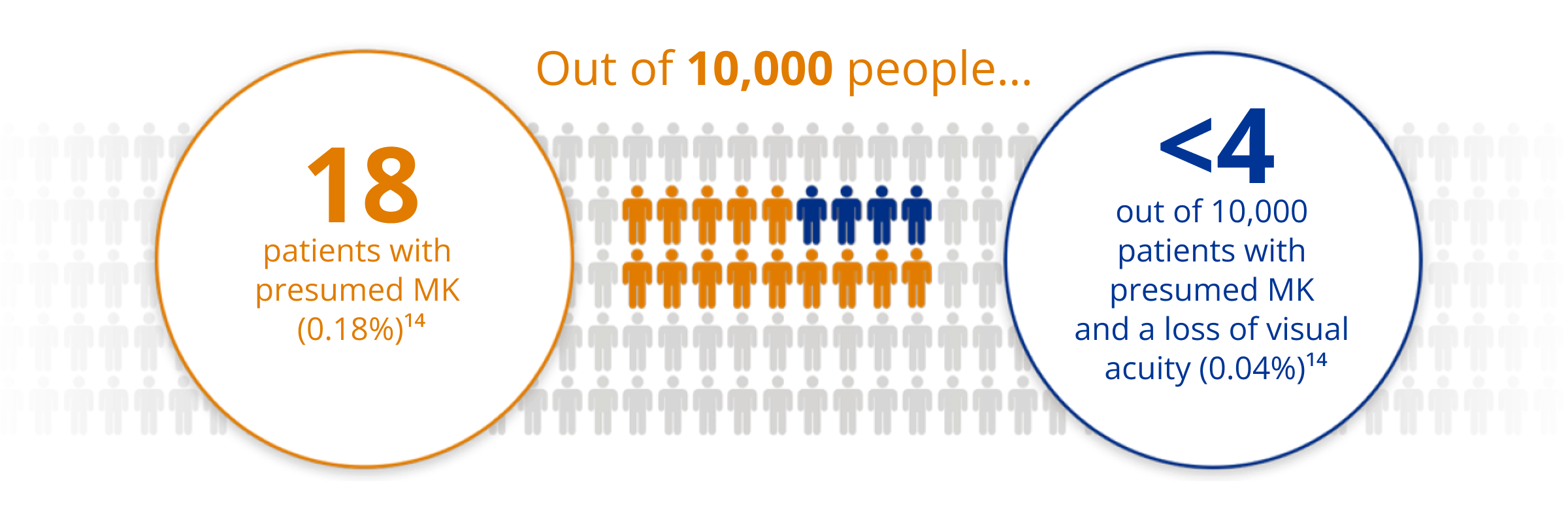
For those who were able to continuously wear the lenses ≥3 weeks, there was a lower rate of presumed MK than for those with <3 weeks of wear (p=0.02).*
*Based on calculated overall annual rate of MK.
To Help Your Patients Get the Most From Their Contact Lenses, Recommend CLEAR CARE® PLUS and OPTI-FREE® Puremoist®

AIR OPTIX® NIGHT & DAY® AQUA Contact Lenses Product Information

*Therapeutic use requires active oversight by the eye care practitioner. See product instructions for complete wear, care and safety information.
†Extended wear for up to 30 continuous nights, as prescribed by an eye care practitioner.


AIR OPTIX® PLUS HYDRAGLYDE® MULTIFOCAL
Delivers a Combination of Technologies for Consistent Comfort*, Long-Lasting Lens Surface Moisture10 and Seamless Vision at All Distances15


AIR OPTIX® PLUS HYDRAGLYDE® FOR ASTIGMATISM
Designed to provide comfortable, clear vision
^Trademarks are the property of their respective owners.
References:
1. Alcon data on file, 2012.
2. Bergenske P, Long B, Dillehay S et al. Long-term Clinical Results: 3 Years of Up to 30-Night Continuous Wear of Lotrafilcon A Silicone Hydrogel and Daily Wear of Low-Dk/t Hydrogel Lenses. Eye & Contact Lens. 2007;33(2):74-80.
3. Based on the ratio of lens oxygen transmissibilities; Alcon data on file, 2009, 2010.
4. Nash WL, Gabriel MM. Ex vivo analysis of cholesterol deposition for commercially available silicone hydrogel contact lenses using a fluorometric enzymatic assay. Eye Contact Lens. 2014;40(5):277-282.
5. Nash W, Gabriel M, Mowrey-McKee M. A comparison of various silicone hydrogel lenses; lipid and protein deposition as a result of daily wear. Optom Vis Sci. 2010;87: E-abstract 105110.
6. Alcon data on file, 2010.
7. Eiden SB, Davis R, Bergenske P. Prospective study of lotrafilcon B lenses comparing 2 versus 4 weeks of wear for objective and subjective measures of health, comfort, and vision. Eye Contact Lens. 2013;39(4):290-294.
8. Lemp J, Kern J. A comparison of real time and recall comfort assessments. Optom Vis Sci. 2016;93:E-abstract 165256.
9. Alcon data on file, 2019.
10. In vitro measurement of contact angles on unworn spherical lenses; significance demonstrated at the 0.05 level; Alcon data on file, 2009.
11. Based on published manufacturer-provided DK/T values in Tylers Quarterly, 2019.
12. Acuvue^ Technical Specifications. Winter 2022 Update. https://www.jnjvisionpro.ca/sites/ca/files/public/content/en-CA/products/tech%20specs/acuvue_tech_specs_october_2022-en.pdf.
13. Biofinity Energys^. https://coopervision.com/practitioner/our-products/biofinity-family/biofinity-energys.
14. Schein O, McNally J, Katz J, Chalmers R. The incidence of microbial keratitis among wearers of a 30-day silicone hydrogel extended-wear contact lens. Ophthalmol. 2005;112(12):2172-79.
15. Alcon data on file, 2008.
Important safety information
See product instructions for complete wear, care and safety information.